Highlights
- We examine the impact of antiplatelet treatment on bleeding after DES implantation.
- Dual antiplatelet treatment will increase overall and minor bleeding.
- Dual antiplatelet treatment is not associated with major bleeding complications.
- Bleeding is not associated with major adverse cardiovascular events.
- Smoking may have a significant role in the occurrence of bleeding.
Abstract
Background
Dual antiplatelet treatment (DAPLT) for at least 12 months is recommended after drug eluting stent (DES) implantation, but concerns regarding the extended use of this treatment persist due to increased risk of bleeding. In this study are assessed the incidence, correlates, and clinical significance of bleeding complications in diabetic patients after long-term DAPLT post DES implantation.
Methods
We studied 610 consecutive diabetic patients after DES implantation. The primary end point was the occurrence of any bleeding according to the BARC and TIMI definitions.
Results
The incidence of overall bleeding was higher in patients on DAPLT (21.1% vs. 4.4%, p < 0.001); minor/minimal according to the TIMI definition, and type 1 or 2 according to the BARC definition, were more frequently observed in patients on DAPLT (20.3% vs. 3.0%, p < 0.001, 15.6% vs. 2.0%, p < 0.001 and 4.4% vs. 0.5%, p = 0.034, respectively), whereas there was no effect on type 3 (3.5% vs. 2.0%, p = ns). DAPLT was an independent predictor for overall (HR 5.35, 95% CI: 2.69–10.67, p < 0.001), minor (HR 7.45, 95% CI: 3.25–17.12, p < 0.001, for TIMI classification) and type 1 or 2 bleeding (HR 8.17, 95% CI 3.29–20.25, p < 0.001); furthermore smoking was also predictor for overall bleeding (HR 1.65, 95% CI: 1.05–2.61, p = 0.030). Cardiovascular adverse events were not more frequent in patients with bleeding as compared with those without bleeding.
Conclusions
Long-term DAPLT in diabetic patients after DES implantation is associated with higher risk of overall and minor but not major bleeding; smoking may have a significant role in the occurrence of bleeding complications.
Keywords
Coronary artery disease;Bleeding;Drug eluting stents;Diabetes mellitus;Antiplatelet therapy
1. Introduction
Antiplatelet agents are used for the treatment and prevention of ischemic events in patients with established atherothrombosis or with risk factors for vascular diseases. Although the early and late benefits of dual antiplatelet treatment (DAPLT) have been demonstrated in multiple studies, concerns regarding the extended use of DAPLT persist. The guidelines recommend continuing DAPLT for over 12 months after implantation of a drug-eluting stent (DES) in patients at low risk of bleeding [1]. Although effective, DAPLT increase the risk of bleeding and bleeding events are associated with worse short- and long-term outcomes [2].
Diabetes mellitus is considered as a major risk factor for cardiovascular events, and coronary thrombosis is more often observed in such patients, as this condition is characterized by elevated concentrations of procoagulant factors which are up-regulated, contributing to a prothrombotic status [3]. Diabetic patients (DP) have an increased risk for restenosis and their clinical follow-up (FU) is often characterized by a higher incidence of death, myocardial infarction (MI) and revascularization [4] ; [5]. For these reasons, it is not surprising that DAPLT in DP is often maintained for more than 12 months, exposing them to a potential increased risk of bleeding complications.
Analyses of bleeding risk among high-risk vascular patients taking antiplatelet therapy for up to 1 year have been reported, and risk factors have been identified [6]. In this study, we assessed the frequency, correlates, and clinical significance of bleeding complications, as well as the outcome regarding major adverse cardiovascular events (MACE), in DP treated with long-term DAPLT after DES implantation.
2. Patients and methods
In this single center prospective registry, 610 consecutive DP (male 80%, mean age 65 ± 9 years) underwent PCI from September 1st, 2003 to June 30, 2006 with 1st generation DES implantation [80% sirolimus-eluting (CYPHER, Jonhnson and Johnson Co), 10% paclitaxel-eluting (TAXUS, Boston Scientific Co), and 10% combined] in their native coronary arteries or aorto-coronary bypass grafts. Eligible patients had a history of stable or unstable coronary artery disease, including acute MI, and a manifest diabetes mellitus treated with oral antidiabetic agent, insulin or both; PCI was performed to treat de novo or restenotic lesions. Major exclusion criteria were hemorrhagic diathesis, known use of warfarin, contraindications to the use of aspirin and thienopyridines, and coexisting conditions that limited life expectancy to less than 12 months.
All patients were treated with oral aspirin (at least 100 mg daily) and clopidogrel (a loading dose of 600 mg was given as soon as possible during the procedure for those without pretreatment, followed by 75 mg daily). Patients were advised to maintain DAPLT for at least 12 months; the use of DAPLT for a longer period was encouraged and left to the physicians discretion.
3. Follow-up, endpoints and definitions
Five years clinical FU was obtained in 587/610 (96%) patients. Clinical outcomes and bleeding complications during FU were obtained by clinical visits in the hospital or serial direct telephone contact (every 12 months) by research fellows of our Cardiology Department and entered into a dedicated database; in case of hospitalization full data from hospital records were received. During the FU period, 89% of patients received DAPLT for 12 months, 68% for 2 years, 64% for 3 years, 59% for 4 years and 56% for 5 years; furthermore 376 (64%) patients remained continuously on DAPLT, whereas 211 discontinued one of the two antiplatelet regiments. There were no differences in demographics, clinical and procedural characteristics in patients on DAPLT vs. single antiplatelet treatment (SAPLT) (Table 1).
| DAPLT N = 376 | SAPLT N = 211 | |||
|---|---|---|---|---|
| N (%) | N (%) | HR (95% CI) | p | |
| Age (years), mean (SD) | 65.1 (8.9) | 64.7 (9.4) | 1.01(0.99–1.02) | 0.094 |
| Age > 65 years | 200(53.2) | 108(51.2) | 1.13(0.93–1.39) | 0.223 |
| Men | 307(81.6) | 164(77.7) | 0.91(0.70–1.18) | 0.494 |
| Hypercholesterolemia | 348 (92.6) | 195 (92.4) | 1.01 (0.69–1.49) | 0.943 |
| Hypertension | 296 (78.7) | 167 (79.1) | 0.99 (0.78–1.27) | 0.982 |
| Smoking | 226 (60.1) | 132 (62.6) | 0.98 (0.80–1.21) | 0.900 |
| IDDM | 81 (21.5) | 41 (19.4) | 1.01 (0.89–1.14) | 0.924 |
| Previous MI | 146 (38.8) | 98 (46.4) | 0.92 (0.75–1.14) | 0.460 |
| Previous CABG | 62 (16.5) | 36 (17.1) | 0.99 (0.75–1.30) | 0.955 |
| ACS at presentation | 136 (36.2) | 65 (30.8) | 1.23 (0.99–1.52) | 0.052 |
| EF < 40% | 36 (9.6) | 24 (11.4) | 0.94 (0.56–1.58) | 0.825 |
| Multivessel disease | 259 (68.9) | 146 (69.2) | 1.03 (0.83–1.29) | 0.742 |
| Multivessel stenting | 71 (18.9) | 33 (15.6) | 1.10 (0.85–1.42) | 0.462 |
| Complete revascularization | 170 (45.2) | 87 (41.2) | 1.04 (0.85–1.27) | 0.700 |
| Number of stent/patient, median (IQR) | 1 (1,2) | 1 (1,2) | 1.03 (0.92–1.17) | 0.576 |
| Total stent length (mm), median (IQR) | 23 (18–36) | 23 (18–33) | 1.00 (0.99–1.01) | 0.697 |
IDDM: insulin dependent diabetes mellitus; MI: myocardial infarction; CABG: coronary artery bypass graft; ACS: acute coronary syndrome; EF: ejection fraction.
The primary study end point was the occurrence of any bleeding according to TIMI [7] or Bleeding Academic Research Consortium [8] (BARC) definitions at clinical FU. We also assessed the MACE, defined as the combination of all-cause mortality/non-fatal MI/cerebrovascular accident (CVA) and the occurrence of definite/probable stent thrombosis according to the Academic Research Consortium (ARC) classification [9].
4. Statistical analysis
Continuous variables are presented with mean and standard deviation (SD) or with median and interquartile range (IQR). Quantitative variables are presented with absolute and relative frequencies. Life table analyses were used to calculate cumulative free bleeding rate for specific time intervals. Event rates were calculated dividing the number of events occurring during the study period by the number of person-years of observation. Incidence rates were presented as the number of events/1000 person-years. The association of each study variable with bleeding was assessed by univariate Cox regression analysis. Variables that showed significant association with the outcome were included in the multivariate Cox proportional-hazard model in a stepwise method (p for removal was set at 0.1 and p for entry was set at 0.05) in order to determine the independent factors for bleeding. Hypothesized interactions of variables in the models were not significant. The assumption of proportional hazards was evaluated by testing for the interaction with a continuous time variable. Hazard ratios (HR) with their 95% Confidence Interval (CI) were also computed to evaluate the association of clinical FU variables (stent thrombosis, death, non-fatal MI, CVA) with bleeding and antiplatelet treatment. Kaplan–Meier estimates for bleeding events were constructed over the FU period. All p values reported are two-tailed. Statistical significance was set at 0.05 and analyses were conducted using STATA statistical software (version 9.0).
5. Results
5.1. Bleeding
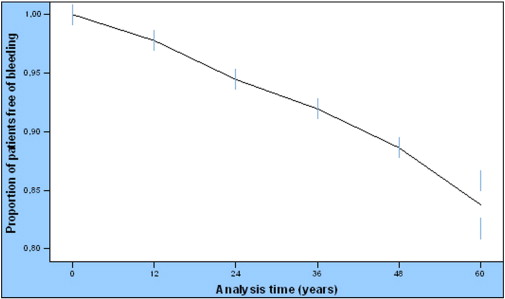
Two (0.3%) major periprocedural bleeding events according to TIMI definition were observed; these events were excluded from further analysis. Among the 587 patients with complete FU data, 90 (15.3%) presented any bleeding event over a median of 30 months; seven out of ninety (7.8%) were classified as having a major bleeding and 83 (92.2%) minor/minimal bleeding according to TIMI definition. According to BARC definition, 10.8% (n = 60) of the study patients had type 1 bleeding and 2.9% (n = 15) for each, type 2 and type 3 bleeding (type 3a 1.6%, type 3b 1.2% and type 3c 0.2%). The most common type of bleeding was upper gastrointestinal (32.2%), followed by bruising (18.4%), hematuria (11.5%), nasal (6.9%), lower gastrointestinal (4.6%), and intraocular (3.4%); intracranial bleeding occurred in 1.1% and other types of bleeding such as gingival, menorrhagia, hemoptysis, in 21.9%. The bleeding event rate/1000 patient-years were 34.85 (95% CI: 28.4–42.7). Cumulative bleeding-free rate for the first year was 97.8% [standard error (SE) 0.6%], for two years 94.5% (SE = 0.9%) and for five years 85.9% (SE = 1.5%) (Fig. 1).
|
|
|
Fig. 1. Cumulative bleeding free rates during the follow-up period. Cumulative bleeding-free rate for the first year was 98.0% (SE = 0.6%), for two years 95.3% (SE = 0.9%) and for five years 89.4% (SE = 1.3%). |
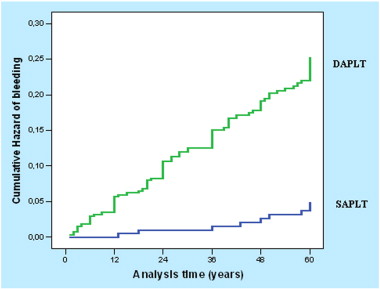
Demographics, clinical and procedural characteristics of all patients and in association with bleeding are presented in Table 2; acute coronary syndromes and smoking had a significant role for bleeding complications (HR 1.67, 95% CI 1.10–2.53, p = 0.015 and HR 1.57, 95% CI 0.99–2.48, p = 0.050 respectively). The results, when the association of demographics, clinical and procedural characteristics with major or minor/minimal bleeding according to TIMI classification was assessed, are shown in Table 3. There were also no differences in the baseline characteristics between those without bleeding and those with type 1, 2 or 3 bleeding according to BARC classification, except of acute coronary syndromes and smoking which were more frequent in type 2 and type 3 bleeding respectively (Table 4). During the total FU period any bleeding event occurred in 21.1% of patients on DAPLT and in 4.4% of patients on SAPLT (HR 5.33, 95% CI: 2.68–10.61, p < 0.001), (Fig. 2); all patients on SAPLT were either on aspirin (66%) or clopidogrel (34%) treatment at the time of the event. Twenty one patients (3.6%) had bleeding complication during the first year of FU and all of them were on DAPLT. Twenty of them were classified as having a minor bleeding according to TIMI definition while 17 as having bleeding type 1 or 2 according to the BARC definition. Excluding patients with bleeding event or death within the first year, 16.9% of patients on DAPLT had bleeding complications vs. 4.5% of patients on SAPLT (HR 4.07, 95% CI: 2.02–8.21, p < 0.001) during the FU period. The bleeding event rate/1000 patient-years was 49.92 (95% CI: 40.4–61.6) for patients on DAPLT and 9.3 (95% CI: 4.9–17.7) for patients on SAPLT. Cumulative bleeding-free rate for the first year was 96.6% (SE = 0.9%) for patients on DAPLT and 100.0% for patients on SAPLT. Bleeding was the cause of permanent DAPLT discontinuation in 56% of patients with such a complication.
| Total (N = 587) | No bleeding (Ν = 497) | Bleeding (Ν = 90) | |||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | HR (95% CI) | p | |
| Age (years), mean (SD) | 65.0 (9.1) | 65.0 (9.2) | 64.7 (8.1) | 1.00 (0.98–1.02) | 0.998 |
| Age > 65 years | 308 (52.5) | 262 (52.7) | 46 (51.1) | 0.99 (0.65–1.49) | 0.959 |
| Men | 471 (80.2) | 400 (80.5) | 71 (78.9) | 1.11 (0.67–1.84) | 0.689 |
| Hypercholesterolemia | 543 (92.5) | 459 (92.4) | 84 (93.3) | 1.15 (0.50–2.63) | 0.742 |
| Hypertension | 463 (78.9) | 393 (79.1) | 70 (77.8) | 0.94 (0.57–1.55) | 0.830 |
| Smoking | 358 (61) | 294 (59.2) | 64 (71.1) | 1.57 (0.99–2.48) | 0.050 |
| IDDM | 122 (20.8) | 107 (21.5) | 15 (16.7) | 0.86 (0.65–1.14) | 0.301 |
| Previous MI | 244 (41.6) | 212 (42.7) | 32 (35.6) | 0.78 (0.50–1.20) | 0.270 |
| Previous CABG | 98 (16.7) | 87 (17.5) | 11 (12.2) | 0.68 (0.36–1.28) | 0.238 |
| ACS at presentation | 201 (34.2) | 161 (32.4) | 40 (44.4) | 1.67 (1.10–2.53) | 0.015 |
| EF < 40% | 60 (10.2) | 55 (11.1) | 5 (5.6) | 0.60 (0.24–1.48) | 0.273 |
| Multivessel disease | 405 (69) | 346 (69.6) | 59 (65.6) | 0.85 (0.55–1.32) | 0.487 |
| Multivessel stenting | 104 (17.7) | 85 (17.1) | 19 (21.1) | 1.26 (0.76–2.10) | 0.361 |
| Complete revascularization | 257 (43.8) | 212 (42.7) | 45 (50.0) | 1.31 (0.86–1.98) | 0.199 |
| Number of stent/patient, median (IQR) | 1 (1,2) | 1 (1,2) | 1 (1,2) | 1.06 (0.83–1.36) | 0.615 |
| Total stent length (mm), median (IQR) | 23 (18–36) | 23 (18–36) | 23 (18–35) | 1.00 (0.99–1.02) | 0.390 |
| No bleeding | Minor bleeding | Major bleeding | |||||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | HR (95% CI)a | p | HR (95% CI)b | p | |
| Age (years), mean | 65.0 (9.2) | 65 (8.1) | 61 (7.3) | 0.96 (0.89–1.03) | 0.261 | 1.00 (0.98–1.03) | 0.755 |
| Age > 65 years | 262 (52.7) | 44 (53.0) | 2 (28.6) | 0.39 (0.07–2.00) | 0.259 | 1.06 (0.69–1.63) | 0.787 |
| Men | 400 (80.5) | 66 (79.5) | 5 (71.4) | 1.65 (0.32–8.54) | 0.546 | 1.07 (0.63–1.82) | 0.802 |
| Dyslipidemia | 459 (92.4) | 78 (94.0) | 6 (85.7) | 0.50 (0.06–4.11) | 0.516 | 1.27 (0.51–3.13) | 0.605 |
| Hypertension | 393 (79.1) | 66 (79.5) | 4 (57.1) | 0.36 (0.08–1.63) | 0.187 | 1.03 (0.61–1.76) | 0.893 |
| Smoking | 294 (59.2) | 57 (68.7) | 6 (85.7) | 3.97 (0.47–32.98) | 0.202 | 1.42 (0.89–2.25) | 0.140 |
| IDDM | 107 (21.5) | 15 (18.1) | 1 (14.3) | 0.18 (0.04–8.82) | 0.392 | 0.90 (0.68–1.19) | 0.475 |
| Previous MI | 212 (42.7) | 29 (34.9) | 3 (42.9) | 1.04 (0.23–4.64) | 0.959 | 0.76 (0.49–1.20) | 0.247 |
| Previous CABG | 87 (17.5) | 10 (12.0) | 1 (14.3) | 0.79 (0.09–6.61) | 0.833 | 0.67 (0.35–1.30) | 0.240 |
| ACS | 161 (32.4) | 37 (44.6) | 3 (42.9) | 1.61 (0.36–7.21) | 0.531 | 1.68 (1.09–2.60) | 0.018 |
| EF < 40% | 55 (11.1) | 5 (6.0) | 0 | 0.04 (0–398.2) | 0.590 | 0.65 (0.26–1.61) | 0.354 |
| Multivessel disease | 346 (69.6) | 56 (67.5) | 3 (42.9) | 0.33 (0.07–1.50) | 0.154 | 0.93 (0.58–1.47) | 0.755 |
| Multivessel stenting | 85 (17.1) | 18 (21.7) | 1 (14.3) | 0.81 (0.09–6.71) | 0.844 | 1.31 (0.77–2.20) | 0.314 |
| Complete revascularization | 212 (42.7) | 41 (49.4) | 4 (57.1) | 1.77 (0.39–7.91) | 0.454 | 1.28 (0.83–1.97) | 0.261 |
| Number of stent/patient, median (IQR) | 1 (1,2) | 1 (1–2) | 1 (1–2) | 0.80 (0.28–2.31) | 0.679 | 1.08 (0.84–1.40) | 0.534 |
| Total stent length (mm), median (IQR) | 23 (18–36) | 23 (18–35) | 33 (23–36) | 1.00 (0.96–1.05) | 0.755 | 1 (0.99–1.02) | 0.422 |
a. Hazard ratio (95% Confidence Interval) for major bleeding.
b. Hazard ratio (95% Confidence Interval) for minor bleeding.
| Type 1 | Type 2 | Type 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | HR (95% CI)a | p | HR (95% CI)b | p | HR (95% CI)c | p | |
| Age (years), mean | 65 (8.5) | 63 (7.2) | 63 (7.0) | 1.01 (0.98–1.04) | 0.431 | 0.98 (0.93–1.03) | 0.403 | 0.98 (0.93–1.03) | 0.491 |
| Age > 65 years | 33 (55.0) | 7 (46.7) | 6 (40) | 1.15 (0.69–1.92) | 0.582 | 0.82 (0.30–2.28) | 0.714 | 0.63 (0.22–1.79) | 0.391 |
| Men | 47 (78.3) | 12 (80) | 12 (80) | 1.13 (0.61–2.10) | 0.681 | 1.04 (0.29–3.70) | 0.946 | 1.04 (0.29–3.68) | 0.953 |
| Dyslipidemia | 55 (91.7) | 14 (93.3) | 14 (93.3) | 0.91 (0.36–2.27) | 0.841 | 1.16 (0.15–8.85) | 0.883 | 1.15 (0.15–8.77) | 0.890 |
| Hypertension | 46 (76.7) | 13 (86.7) | 11 (73.3) | 0.88 (0.48–1.59) | 0.670 | 1.73 (0.39–7.70) | 0.467 | 0.75 (0.24–2.35) | 0.621 |
| Smoking | 38 (63.3) | 12 (80) | 14 (93.3) | 1.13 (0.67–1.91) | 0.645 | 2.65 (0.75–9.39) | 0.131 | 9.26 (1.21–70.42) | 0.032 |
| IDDM | 11 (18.3) | 2 (13.3) | 2 (13.3) | 0.90 (0.65–1.25) | 0.545 | 0.75 (0.35–1.58) | 0.448 | 0.75 (0.36–1.58) | 0.454 |
| Previous MI | 22 (36.7) | 5 (33.3) | 5 (33.3) | 0.82 (0.48–1.39) | 0.461 | 0.69 (0.24–2.03) | 0.505 | 0.70 (0.24–2.03) | 0.508 |
| Previous CABG | 8 (13.3) | 0 | 3 (20) | 0.74 (0.35–1.56) | 0.435 | 0.04 (0.00–12.74) | 0.269 | 1.20 (0.34–4.25) | 0.778 |
| ACS | 24 (40.0) | 9 (60) | 7 (46.7) | 1.42 (0.85–2.38) | 0.183 | 3.13 (1.11–8.81) | 0.030 | 1.86 (0.67–5.15) | 0.228 |
| EF < 40% | 4 (6.7) | 1 (6.7) | 0 | 0.72 (0.26–1.99) | 0.232 | 0.67 (0.09–5.09) | 0.698 | 0.04 (0.0–84.85) | 0.416 |
| Multivessel disease | 38 (63.3) | 11 (73.3) | 10 (66.7) | 0.79 (0.46–1.33) | 0.373 | 1.20 (0.38–3.78) | 0.750 | 0.89 (0.30–2.61) | 0.837 |
| Multivessel stenting | 12 (20.0) | 4 (26.7) | 3 (20) | 1.18 (0.62–2.22) | 0.610 | 1.74 (0.55–5.48) | 0.340 | 1.21 (0.34–4.28) | 0.770 |
| Complete revascularization | 31 (51.7) | 7 (46.7) | 7 (46.7) | 1.38 (0.83–2.29) | 0.210 | 1.17 (0.42–3.23) | 0.759 | 1.61 (0.42–3.20) | 0.773 |
| Number of stent/patient, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1.06 (0.78–1.43) | 0.716 | 0.96 (0.50–1.85) | 0.920 | 1.17 (0.66–2.07) | 0.590 |
| Total stent length (mm), median (IQR) | 23 (18–35) | 23 (18–34) | 26 (18–36) | 1.00 (0.99–1.02) | 0.438 | 0.99 (0.96–1.03) | 0.897 | 1.01 (0.98–1.04) | 0.495 |
a. Hazard ratio (95% Confidence Interval) for type 1 bleeding.
b. Hazard ratio (95% Confidence Interval) for type 2 bleeding.
c. Hazard ratio (95% Confidence Interval) for type 3 bleeding.
|
|
|
Fig. 2. Cumulative hazard of bleeding for patients on DAPLT vs. SAPLT treatment. Patients on DAPLT had greater hazard for bleeding complication. DAPLT: dual antiplatelet treatment, SAPLT: single antiplatelet treatment. |
Major according to TIMI definition, or type 3 bleeding (Table 5), showed no significant different hazard between patients on DAPLT or SAPLT; on the contrast, a significant association of DAPLT with bleeding types 1 and 2, such as minor/minimal bleeding complications according to TIMI classification was observed. When multiple Cox proportional hazard analysis in a stepwise method was conducted, DAPLT treatment, smoking and acute coronary syndromes were predictors for overall bleeding complications (HR 5.35, 95% CI: 2.69–10.67, p < 0.001, HR 1.65, 95% CI: 1.05–2.61, p = 0.030 and HR 1.63, 95% CI: 1.07–2.47, p = 0.021 respectively); DAPLT treatment and acute coronary syndromes were also predictors for bleeding type 1 or 2 (HR 8.17, 95% CI 3.29–20.25, p < 0.001and HR 1.58, 95% CI 1.00–2.50, p = 0.048 respectively) and minor/minimal bleeding according to TIMI definition(HR 7.45, 95% CI: 3.25–17.12, p < 0.001and HR 1.61, 95% CI: 1.05–2.49, p = 0.030 respectively).
| DAPLT N (%) | SAPLT N (%) | HR (95% CI) | p | |
|---|---|---|---|---|
| Overall bleeding | 81 (21.1) | 9 (4.4) | 5.33 (2.68–10.61) | < 0.001 |
| Type 3 bleeding | 11 (3.5) | 4 (2.0) | 1.79 (0.57–5.63) | 0.327 |
| Type 2 bleeding | 14 (4.4) | 1 (0.5) | 8.96 (1.18–68.14) | 0.034 |
| Type 1 bleeding | 56 (15.6) | 4 (2.0) | 8.40 (3.05–23.18) | < 0.001 |
| Major (TIMI) | 4 (1.3) | 3 (1.5) | 0.87 (0.19–3.90) | 0.851 |
| Minor/minimal (TIMI) | 77 (20.3) | 6 (3.0) | 7.58 (3.30–17.39) | < 0.001 |
5.2. Clinical events
There was no difference in the incidence of all-cause mortality, non-fatal MI, and CVA in patients on DAPLT vs. SAPLT at FU (12% vs 9%, HR 1.39, 95% CI 0.83–2.32, p = 0.207 for death, 4.2% vs 3.5%, HR 1.23, 95% CI 0.53–2.86, p = 0.617 for non-fatal MI, and 4.2% vs 3.5%, HR 1.24, 95% CI 0.54–2.88, p = 0.606 for CVA); the incidence for MACE was 18.7% and 13.7% respectively (HR 1.41, 95% CI 0.93–2.14, p = 0.10). During the first year of FU, 18 (3%) patients presented MACE, 15 (83.3%) of which were on DAPLT (p = 0.02); 79 patients (13.9%) presented MACE after the 1st year of FU. Patients on DAPLT after the first year of FU did not have significant hazard of MACE as compared with those on SAPLT (17% vs 15.8%, HR 1.1, 95% CI 0.64–1.87, p = 0.73). The cumulative survival rate at 5 years was 88% in patients on DAPLT and 91% on SAPLT. Patients with bleeding complications had no significant different hazard for stent thrombosis, death, non-fatal MI or CVA (Table 6). The incidence of definite/probable stent thrombosis was 2.1% in patients on DAPLT and 0.8% on SAPLT (HR 1.32, 95% CI 0.27–6.58, p = 0.73); all 4 definite and 3 probable stent thrombosis were on DAPLT whereas 2 probable stent thrombosis were on SAPLT. Regarding the time of the events, 1 definite and 1 probable (on DAPLT) occurred on day 7 and day 25 after PCI respectively, 2 definite occurred during months 8 and 11 and 1 probable (on DAPLT) during month 5 after PCI and finally 1 definite and 3 probable stent thrombosis were considered as very late month 54 and months 27, 29, and 40 respectively. During the FU period 127 patients (21.6%) underwent repeat PCI procedure either for target lesion revascularization (n = 20), non-target lesion revascularization due to disease progression (n = 89), or both (n = 18). No significant differences in bleeding events were observed between this group of patients and those without repeat revascularization (HR 1.09, 95% CI 0.68–1.74, p = 0.719).
| Clinical follow-up | No bleeding | Bleeding | ||
|---|---|---|---|---|
| N (%) | N (%) | HR (95% CI) | P | |
| Definite/probable ST | 8 (1.6) | 1 (1.1) | 0.69 (0.08–5.50) | 0.725 |
| Definite | 3 (0.6) | 1 (1.1) | ||
| Probable | 5 (1.0) | 0 | ||
| Death | 56 (11.3) | 7 (7.8) | 0.67 (0.30–1.48) | 0.328 |
| Non-fatal MI | 19 (3.8) | 4 (4.4) | 1.13 (0.38–3.34) | 0.815 |
| CVA | 20 (4.0) | 3 (3.3) | 0.80 (0.24–2.71) | 0.728 |
| All events | 83 (16.7) | 14 (15.6) | 0.91 (0.51–1.60) | 0.747 |
ST: stent thrombosis; MI: myocardial infarction; CVA: cerebrovascular accident.
6. Discussion
This study showed that long-term DAPLT after PCI in DP significantly increases the risk of overall bleeding complications, but this increase is limited to minor bleeding, irrespective the definition is used; this excess risk is steady for the whole period of FU. Except for the use of DAPLT, smoking and acute coronary syndromes were also predictors for overall bleeding, whereas bleeding did not correlate with MACE or definite/probable stent thrombosis. Because the definition clearly plays an important role in terms of the measured incidence of bleeding, two definitions were used in the current study for a better interpretation of the results.
Unlike ischemic clinical events such as cardiac death, MI, or stent thrombosis, there is substantial heterogeneity among the many bleeding definitions currently in use. Current bleeding definitions consist of both laboratory parameters, such as decreases in hemoglobin and hematocrit scores, and clinical events, including the need for transfusion or surgery, cardiac tamponade, hematomas, and various degrees of bleeding. Each definition incorporates a different combination of these data elements and then rank orders these combinations into severity categories, which vary widely between definitions. The Thrombolysis in Myocardial Infarction (TIMI) bleeding criteria have been in use for nearly 30 years, and have been reported in most cardiovascular trials. The need to develop and standardize bleeding end-point definitions led the Bleeding Academic Research Consortium to propose new objective, hierarchically graded, consensus classification for bleeding.
In this study, regardless of the definition used, the severity of bleeding was almost similar, as about 86% of patients defined as having minor/minimal bleeding according to TIMI definition presented as also having type 1 or 2 bleeding according to BARC definition.
The prolong use of DAPLT results in markedly reduced risk of thrombotic events associated with stent implantation but concerns regarding the extended use of DAPLT persist, mainly due to the bleeding risk. The incidence of increased bleeding with DAPLT has been well examined in several studies. In the CURE trial, clopidogrel and aspirin therapy was associated with a significant increase in major and minor bleeding compared with aspirin alone, but no increase in life-threatening bleeding events was observed [10]. Similarly, in a meta-analysis of 18 randomized trials, DAPLT therapy was associated with a significantly increased risk of major and minor bleeding events compared to single agent therapy; [11] although based on small numbers, there were no significant differences in fatal or intracranial bleedings. In the CHARISMA trial the rate of severe bleeding (according to GUSTO definition) was not significantly greater with DAPLT, but a significant increase in the incidence of moderate bleeding was observed [12]. In the same trial, the risk of moderate or severe bleeding while on DAPLT was greater in the first year, but after this period the bleeding rates were similar in both groups. Generally, in trials evaluating extended treatment with DAPLT major bleeding rates have ranged from ~ 2% to ~ 4% [10]; [12] ; [13].
There were 56 deaths (11.3%) in no bleeding group in this study, and only 7 (7.8%) in bleeding group but this difference was not statistically significant. This is an unusual finding and someone could consider that these patients were “prevented” from having a bleeding event by dying. Several studies have examined the relationship between bleeding, mortality and other MACE. In these studies different definitions of bleeding were used, including not only the TIMI definitions, used in this study, but more than a dozen others [14]. A few definitions have been reported to be associated with mortality or other events such as GUSTO [15] definition in the CHARISMA trial. Less severe bleeding sometimes becomes clinically more important with long term therapies, because minor bleedings may lead to discontinuation of antiplatelet treatment by the patient. Withdrawal of DAPLT has been reported as a cause of stent thrombosis after DES implantation; furthermore, discontinuation of DAPLT seems to be the most potent predictor of the late stent thrombosis, and barring a bleeding contraindication, provides sufficient reason to recommend extended DAPLT in patients treated with DES, particularly those with acute coronary syndrome or diabetes mellitus [16]; [17] ; [18].
On the other hand, several trials [19] ; [20] have shown no significant benefit associated with the extended continuation of DAPLT in reducing the incidence of cardiovascular events; in addition, a significant increase in the number of actionable bleeding episodes has been observed [20]. In a recent meta-analysis of randomized trials, the extension of DAPLT duration increased the risk of TIMI major bleeding without reducing ischemic events [21]. In another study 6-month vs. longer DAPLT duration was not associated with increased likelihood of thrombotic events at 3-year FU, while major bleeding was negligible across groups [22].
Of interest is the observation, that smoking had a significant role in the occurrence of bleeding in our study patients. In a recent study, smoking was found to be a risk factor for serious bleeding [23]. Clopidogrel is metabolically activated by several hepatic cytochrome P450 (CYP) isoenzymes, including CYP1A2; [24] cigarette smoking induces CYP1A2 [25] and may, therefore, enhance the conversion of clopidogrel to its active metabolite, associated with increased platelet inhibition and lower aggregation as compared with nonsmokers [26]. These clinical observations may be explained by the adverse effects that smoking has on mucosal aggressive and protective factors [27].
7. Limitations
This is a single center non-randomized prospective study with the decision about the duration of DAPLT after 12 months determined by the physicians' discretion. Duration of DAPLT was widely variable, and hidden compounders may be impossible to adjust; however there were no differences on demographics and clinical or procedural characteristics in patients on DAPLT vs. SAPLT treatment at any time period after 12 months.
The study was conducted when only 1st generation DES were available, so the results could be considered as less relevant to current day PCI-practice with newer DES designs. Furthermore, diabetic patients often remain on DAPLT for a long period of time as a high risk subset of patients or due to extensive cardiovascular disease.
Although clinical FU was completed in 96% of patients, there is still a risk of under as well as over reporting of adverse events. Different definitions of bleeding, other than TIMI or BARC definition used in this study, could lead to different results about the safety of long-term antiplatelet treatment.
8. Conclusions
Prolong DAPLT increases the risk for bleeding (especially minor, according to TIMI definition and type 1 or 2 according to BARC definitions) in DP, and this excess risk is steady for the whole period of clinical FU. Bleeding complications are not associated with increased risk of definite/probable stent thrombosis or MACE. Acute coronary syndromes and smoking may have a significant role in the occurrence of bleeding in DP on DAPLT, but this observation needs further investigation.
Conflict of interest
There is no conflict of interest to declare.
References
- [1] S.B. King, S.C. Smith, J.W. Hirshfeld, A.K. Jacobs, D.A. Morrison, D.O. Williams, et al.; 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee; Circulation, 117 (2008), pp. 261–295
- [2] J.W. Eikelboom, S.R. Mehta, S.S. Anand, C. Xie, K.A. Fox, S. Yusuf; Adverse impact of bleeding on prognosis in patients with acute coronary syndromes; Circulation, 114 (2006), pp. 774–782
- [3] P.R. Moreno, V. Fuster; New aspects in the pathogenesis of diabetic atherothrombosis; J Am Coll Cardiol, 44 (2004), pp. 2293–2300
- [4] A.J. Scheen, F. Warzee, V.M. Legrand; Drug-eluting stents: meta-analysis in diabetic patients; Eur Heart J, 25 (2004), pp. 2167–2168
- [5] T.H. Yang, S.W. Park, M.K. Hong, D.W. Park, K.M. Park, Y.H. Kim, et al.; Impact of diabetes mellitus on angiographic and clinical outcomes in the drug-eluting stents era; Am J Cardiol, 96 (2005), pp. 1389–1392
- [6] S.R. Steinhubl, P.B. Berger, J.T. Mann, E.T. Fry, A. DeLago, C. Wilmer, et al.; Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial; JAMA, 288 (2002), pp. 2411–2420
- [7] J.H. Chesebro, G. Knatterud, R. Roberts, J. Borer, L.S. Cohen, J. Dalen, et al.; Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge; Circulation, 76 (1987), pp. 142–154
- [8] R. Mehran, S.V. Rao, D.L. Bhatt, C.M. Gibson, A. Caixeta, J. Eikelboom, et al.; Standardized Bleeding Definitions for Cardiovascular Clinical Trials. A Consensus Report From the Bleeding Academic Research Consortium; Circulation, 123 (2011), pp. 2736–2747
- [9] D.E. Cutlip, S. Windecker, R. Mehran, A. Boam, D.J. Cohen, G.A. van Es, et al.; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions; Circulation, 115 (2007), pp. 2344–2351
- [10] S. Yusuf, F. Zhao, S.R. Mehta, S. Chrolavicius, G. Tognoni, K.K. Fox, et al.; Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation; N Engl J Med, 345 (2001), pp. 494–502
- [11] V.L. Serebruany, A.I. Malinin, J.J. Ferguson, J. Vahabi, D. Atar, C.H. Hennekens; Bleeding risks of combination vs. single antiplatelet therapy: a meta-analysis of 18 randomized trials comprising 129,314 patients; Fundam Clin Pharmacol, 22 (2008), pp. 315–321
- [12] D.L. Bhatt, K.A. Fox, W. Hacke, P.B. Berger, H.R. Black, W.E. Boden, for the CHARISMA Investigators, et al.; Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events; N Engl J Med, 354 (2006), pp. 1706–1717
- [13] S.D. Wiviott, E. Braunwald, C.H. McCabe, G. Montalescot, W. Ruzyllo, S. Gottlieb, TRITON-TIMI 38 Investigators, et al.; Prasugrel versus clopidogrel in patients with acute coronary syndromes; N Engl J Med, 357 (2007), pp. 2001–2015
- [14] S.R. Steinhubl, A. Kastrati, P.B. Berger; Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions, and its impact on the apparent safety of antithrombotic drugs; Am Heart J, 154 (2007), pp. 3–11
- [15] The GUSTO Investigators; An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction; N Engl J Med, 329 (1993), pp. 673–682
- [16] E.P. McFadden, E. Stabile, E. Regar, E. Cheneau, A.T. Ong, T. Kinnaird, et al.; Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy; Lancet, 364 (2004), pp. 1519–1521
- [17] I. Iakovou, T. Schmidt, E. Bonizzoni, L. Ge, G.M. Sangiorgi, G. Stankovic, et al.; Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents; JAMA, 293 (2005), pp. 2126–2130
- [18] M. Pfisterer, H.P. Brunner-La Rocca, P.T. Buser, P. Rickenbacher, P. Hunziker, C. Mueller, et al.; Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents; J Am Coll Cardiol, 48 (2006), pp. 2584–2591
- [19] S.J. Park, D.W. Park, Y.H. Kim, S.J. Kang, S.W. Lee, C.W. Lee, et al.; Duration of dual antiplatelet therapy after implantation of drug-eluting stents; N Engl J Med, 362 (2010), pp. 1374–1382
- [20] M. Valgimigli, G. Campo, M. Monti, P. Vranckx, G. Percoco, C. Tumscitz, Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators, et al.; Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial; Circulation, 125 (2012), pp. 2015–2026
- [21] S. Cassese, R.A. Byrne, T. Tada, L.A. King, A. Kastrati; Clinical impact of extended dual antiplatelet therapy after percutaneous coronary interventions in the drug-eluting stent era: a meta-analysis of randomized trials; Eur Heart J (Oct. 22 2012) https://doi.org/10.1093/eurheartj/ehs318 [Epub ahead of print]
- [22] D.E. Kandzari, C.S. Barker, M.B. Leon, L. Mauri, W. Wijns, J. Fajadet, et al.; Dual Antiplatelet Therapy Duration and Clinical Outcomes Following Treatment With Zotarolimus-Eluting Stents; J Am Coll Cardiol Cardiovasc Interv, 4 (2011), pp. 1119–1128
- [23] M.J. Alberts, D.L. Bhatt, S.C. Smith, J. Röther, S. Goto, A.T. Hirsch, for the REACH Registry Investigators, et al.; Risk factors and outcomes for patients with vascular disease and serious bleeding events; Heart, 97 (2011), pp. 1507–1512
- [24] J.T. Brandt, S.L. Close, S.J. Iturria, C.D. Payne, N.A. Farid, C.S. Ernest II, et al.; Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel; J Thromb Haemost, 5 (2007), pp. 2429–2436
- [25] S. Zevin, N.L. Benowitz; Drug interactions with tobacco smoking; Clin Pharmacokinet, 36 (1999), pp. 425–438
- [26] K.P. Bliden, J. Dichiara, L. Lawal, A. Singla, M.J. Antonino, B.A. Baker, et al.; The association of cigarette smoking with enhanced platelet inhibition by clopidogrel; J Am Coll Cardiol, 12 (52) (2008), pp. 531–533
- [27] G.L. Eastwood; Is smoking still important in the pathogenesis of peptic ulcer disease?; J Clin Gastroenterol, 25 (Suppl. 1) (1997), pp. S1–S7
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?