Dear Editor,
Escitalopram has been shown to have better efficacy and safety profile than other selective serotonin reuptake inhibitor and serotonin norepinephrine reuptake inhibitor drugs. Clinical studies have shown that it is effective and well tolerated in the treatment of depression and anxiety disorders. Only a few cases of severe skin reaction have been reported. Herein we report one case of telangiectasia in a photoexposed distribution following use of escitalopram. Iatrogenic telangiectasia is a poorly understood dermatological side-effect secondary to the administration of many drugs and the role of photo-exposition as trigger has also been described.
Cutaneous side effects are among the most common observed during treatment with psychotropic drugs: their frequency is estimated to be almost double than with any other class of pharmaceutical molecules. In consideration of the wide and increasing use of antidepressants, knowledge of the adverse events – particularly the most serious ones – related to these drugs is important for dermatologists and allergists.1
Escitalopram is a commonly used antidepressant belonging to the family of selective serotonin reuptake inhibitors (SSRIs). Typical therapeutic doses are 10 and 20 mg, although 5 mg are sometimes given as a starting dose or for specific groups of patients. Similarly to other SSRIs, escitalopram achieves its action by inhibiting uptake of 5-hydroxytryptamine, thus increasing the extracellular levels of this neurotransmitter,2 but is more efficacious and safe than other members of the same class, including racemic citalopram,3 and this explains its diffusion for the treatment of depression and anxiety disorders. Known adverse events include diarrhea, dizziness, dry mouth, fatigue, headache, nausea, sexual dysfunction, sweating, tremor, and weight gain.4 Escitalopram is metabolized by the cytochrome P 450 (CYP) isoenzymes CYP3A4 and CYP2C19 (and, to a lesser extent, CYP2D6); inhibition of other CYP enzymes or P-glycoprotein is limited, and, consequently, the potential for interaction with other drugs is low.5
To the best of our knowledge, telangiectasia in a photoexposed distribution after use of escitalopram was not previously reported.
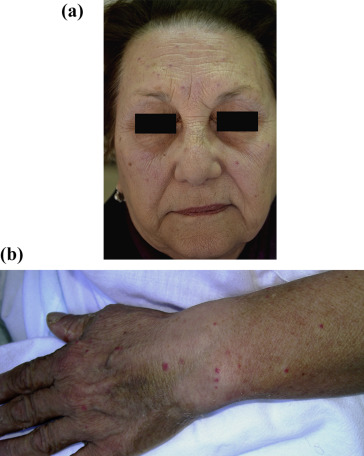
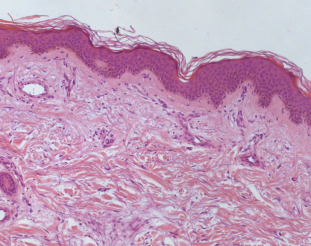
An otherwise healthy 81 year-old woman was referred to our hospital because of a four days history of telangiectatic skin lesions. She had started oral escitalopram (10 mg daily) eleven days earlier, for a major depressive disorder. She was not using other systemic or topical medications, and had no history of photosensitivity, rosacea or application of topical steroids. Physical examination showed several asymptomatic telangiectasias and angiomatous-like macules and papules, widely distributed on face, forearms and dorsum of both hands (Fig. 1a, b). Laboratory tests (liver function tests, ANA, ENA, Scl-70 antibody and ANCA) were within normal values. Histology showed normal epidermis; blood vessels in the superficial dermis were prominent and focally dilated. Mild lymphocytic infiltration with rare eosinophils was present (Fig. 2). Since distribution in photoexposed areas suggested a light-induced adverse reaction, photo testing was performed. Patch tests were performed with standard photoallergens and 5% and 10% escitalopram in petrolatum, with negative results; photo-patch test with the same substances and a single suberythematous dose of 30 J/cm2 UVA on the upper back produced telangiectatic lesions at 28 h with escitalopram only (both 5% and 10%), providing evidence of a photo-induced drug reaction.
|
|
|
Fig. 1. (a) Telangiectasia widely distributed on the face. (b) Forearms and on the dorsa of both hands. A permission of the photograph use was obtained from the patient. |
|
|
|
Fig. 2. Epidermis was normal. Blood vessels in the superficial dermis were prominent and focally dilated. Mild lymphocytic infiltration with rare eosinophils was present (hematoxylin and eosin stain). |
Because of the relationship between administration of escitalopram and onset of the telangiectasia, confirmed by phototesting, the drug was immediately stopped and the patient was treated with oral methylprednisolone (16 mg/day with progressive reduction) and oral nicotinamide (500 mg/day). Gradual and progressive improvement of cutaneous manifestations was observed, until complete resolution, which occurred in one month. Re-challenge with escitalopram was not performed because refused by the patient. Subsequent photoexposure, as well as a photoprovocation test with up to 50 J/cm2 UVA and up to 85 mJ/cm2 UVB (minimal erythema doses of the patient), did not elicit any reaction.
Iatrogenic telangiectasia is a poorly understood dermatological undesired effect of several drugs, like the calcium-antagonists amlodipine and felodipine, lithium, thiothixene, interferon-alpha, venlafaxine and isotretinoin. In some cases, iatrogenic telangiectasia can be triggered by photoexposure: this was described in subjects in treatment with cefotaxime, nifedipine, amlodipine and venlafaxine.6 and 7
Differential diagnoses to be considered are recurrent eruptive pseudoangiomatosis, erythema punctatum of Higuchi (caused by mosquito bites) and eruptive cherry angiomas. In such cases, medical history is particularly important for a correct diagnosis.
In the case presented here, short time to onset of eruptive telangiectatic lesions after administration of escitalopram, absence of concomitant treatments, progressive resolution after discontinuation of the suspected drugs and positive results of photoprovocation test are definitely suggestive of a photoinduced drug reaction.
Considerable evidence exists about photoprotective effects of oral nicotinamide: it prevents the loss in cellular energy consequent to UV exposure, enabling the immune system and cellular energy processes such as DNA repair, to proceed in an optimal manner. Furthermore, nicotinamide is the sole substrate and an inhibitor of the nuclear enzyme poly-ADP-ribose polymerase 1, which is activated by UV and is involved in DNA repair, genomic stability, and regulation of inflammatory cytokines, chemokines, adhesion molecules and inflammatory mediators.8
Severe cutaneous reactions caused by antidepressants were initially reported only for tricyclic drugs. Although more recent molecules of the same class, such as SSRIs, are more selective and safer, the risk of undesired cutaneous effects still exists, apparently related to individual characteristics of patients, namely selectivity of action of the drug on specific receptors. Elderly age is another important individual risk factor, directly and indirectly (frequent presence of co-morbidities and, consequently, concomitant use of several drugs).
Although classified in the same pharmacological group, SSRIs have different chemical structures and effects, particularly on cytochrome P4509: thus, it appears unlikely that their cutaneous side effects are due to shared chemical characteristics, and a conclusive pathogenetic explanation is currently unavailable. Huang et al. suggested that serotonin released around skin appendages regulates inflammation and immune reactions, and serotonin receptors mediate cutaneous vasoconstriction and stimulate cytokine release from keratinocytes and lymphocytes. 10 Use of escitalopram is associated with a number of mucocutaneous undesired effects; only few cases of photosensitivity were reported in controlled trials and in postmarket surveillance, and none of them was of telangiectasia.4
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1 M. Herstowska, O. Komorowska, W.J. Cubała, K. Jakuszkowiak-Wojten, M. Gałuszko-Węgielnik, J. Landowski; Severe skin complications in patients treated with antidepressants: a literature review; Postepy Dermatol Alergol, 31 (2014), pp. 92–97
- 2 C. Sanchez, E.H. Reines, S.A. Montgomery; A comparative review of escitalopram, paroxetine, and sertraline: are they all alike?; Int Clin Psychopharmacol, 29 (2014), pp. 185–196
- 3 D. Pastoor, J. Gobburu; Clinical pharmacology review of escitalopram for the treatment of depression; Expert Opin Drug Metab Toxicol, 10 (2014), pp. 121–128
- 4 M.V. Mitkov, R.M. Trowbridge, B.N. Lockshin, J.P. Caplan; Dermatologic side effects of psychotropic medications; Psychosomatics, 55 (2014), pp. 1–20
- 5 N. Rao; The clinical pharmacokinetics of escitalopram; Clin Pharmacokinet, 46 (2007), pp. 281–290
- 6 M. Vaccaro, F. Borgia, O. Barbuzza, B. Guarneri; Photodistributed eruptive telangiectasia: an uncommon adverse drug reaction to venlafaxine; Br J Dermatol, 157 (2007), pp. 822–824
- 7 F. Borgia, M. Vaccaro, F. Guarneri, S.P. Cannavo; Photodistributed telangiectasia following use of cefotaxime; Br J Dermatol, 143 (2000), pp. 674–675
- 8 A.C. Chen, D.L. Damian, G.M. Halliday; Oral and systemic photoprotection; Photodermatol Photoimmunol Photomed, 30 (2014), pp. 102–111
- 9 C. Ram-Wolf, E. Mahé, P. Saiag; Escitalopram photo-induced erythroderma; J Eur Acad Dermatol Venereol, 22 (2008), pp. 1015–1017
- 10 J. Huang, G. Li, J. Xiang, D. Yin, R. Chi; Immunohistochemical study of serotonin in lesions of chronic eczema; Int J Dermatol, 43 (2004), pp. 723–726
Document information
Published on 05/04/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?