Abstract
Background
The National Toxicology Program (NTP) performed short-term toxicity studies of tetra- and pentavalent vanadium compounds, vanadyl sulfate and sodium metavanadate, respectively. Due to widespread human exposure and a lack of chronic toxicity data, there is concern for human health following oral exposure to soluble vanadium compounds.
Objectives
To compare the potency and toxicological profile of vanadyl sulfate and sodium metavanadate using a short-term in vivo toxicity assay.
Methods
Adult male and female Harlan Sprague Dawley (HSD) rats and B6C3F1/N mice, 5 per group, were exposed to vanadyl sulfate or sodium metavanadate, via drinking water, at concentrations of 0, 125, 250, 500, 1000 or 2000 mg/L for 14 days. Water consumption, body weights and clinical observations were recorded throughout the study; organ weights were collected at study termination.
Results
Lower water consumption, up to −80% at 2000 mg/L, was observed at most exposure concentrations for animals exposed to either vanadyl sulfate or sodium metavanadate and was accompanied by decreased body weights at the highest concentrations for both compounds. Animals in the 1000 and 2000 mg/L sodium metavanadate groups were removed early due to overt toxicity. Thinness was observed in high-dose animals exposed to either compound, while lethargy and abnormal gait were only observed in vanadate-exposed animals.
Conclusions
Based on clinical observations and overt toxicity, sodium metavanadate appears to be more toxic than vanadyl sulfate. Differential toxicity cannot be explained by differences in total vanadium intake, based on water consumption, and may be due to differences in disposition or mechanism of toxicity.
Keywords
Pentavalent vanadium ; Tetravalent vanadium ; Vanadyl sulfate ; Sodium metavanadate ; B6C3F1/N ; Harlan Sprague-Dawley ; Short-term toxicity ; National Toxicology Program
1. Introduction
Vanadium is a naturally occurring metal, found in several minerals and fossil fuels and exists in various oxidation states from −1 to +5 [5] . It has been estimated that natural processes, such as volcanic eruptions and weathering, result in the release of 65k tons of vanadium into the environment, while anthropogenic sources add an additional 200k tons per year [30] . Anthropogenic sources of vanadium include various industrial processes, e.g. coal-fired power plant production of fly ash and intermediates of iron smelting. In addition to domestic generation of vanadium-containing industrial waste, the United States imports additional waste and intermediates for use in steel production; in 2013 it was reported that more than 9000 t of vanadium (as ferrovanadium, vanadium pentoxide or ash and residues) were imported by the U.S. [22] . Likely due to its industrial uses, vanadium has been detected at several Superfund sites (at least 319 out of 1699) as classified by the EPA [2] .
The National Toxicology Program (NTP) has previously studied the carcinogenic potential of vanadium pentoxide in male and female F344/N rats and B6C3F1/N mice following chronic whole-body inhalation exposure, up to 2 mg/m3 . Exposure to vanadium pentoxide is primarily occupational. Chronic exposure to vanadium pentoxide resulted in increased incidences of several non-neoplastic lesions of the respiratory tract (lung, larynx, nose) and increased incidences of alveolar/bronchiolar neoplasms in male and female mice, and to some degree in male rats [16] . Vanadium pentoxide has since been listed on California’s Proposition 65 list of chemicals “Known to the State [of California] to Cause Cancer or Reproductive Toxicity” and classified as “Possibly Carcinogenic to Humans”, category 2B, by the International Agency for Research on Cancer [9] and [20] .
While occupational exposure to vanadium primarily occurs via inhalation, environmental exposure occurs largely via ingestion of soluble vanadium compounds. Ingestion of vanadium may include dietary supplements, food or contaminated drinking water. Due to being a ubiquitous element, food is considered the primary source of exposure to vanadium. Many foods naturally contain some vanadium, with the amounts varying based on soil/water conditions and the extent of vanadium absorption into the food product. Background consumption of vanadium from food is estimated to be between 10 and 60 μg daily [2] and [15] . Anthropogenic sources of vanadium have the potential to increase human exposure to vanadium through food and drinking water. Vanadium (V) levels in soil from non-contaminated areas range from 3 to 310 μg V/g soil and have been measured up to 400 μg V/g soil in fly ash contaminated areas [30] . Fly ash contamination of drinking water may also contribute to vanadium exposure; in North Carolina, the Department of Environmental and Natural Resources (NC DENR) has led efforts to assess vanadium drinking water contamination due to fly ash leakage. The Coal Ash Management Act of 2014 enforced by the NC DENR has required Duke Energy to test any well within 1500 feet of all Duke Energy coal-fired facilities in the state. Data released in August 2015 list vanadium as an analyte exceeding the N.C. interim maximum allowable concentration (0.3 μg/L) at 10 of the 11 sites, with 2–118 wells tested at each site [19] . In addition, vanadium has been studied by numerous investigators due to reported effects on glucose metabolism; a summary of the therapeutic history of vanadium and diabetes can be found in [28] . Outside of potential pharmaceutics, vanadium is also sold as a dietary supplement with purported benefits including cholesterol metabolism and enhancing athletic performance.
Due to industrial releases of vanadium into the environment and consumption of dietary supplements, there is concern for human health following chronic oral exposure to soluble vanadium salts. The uncertainty regarding safety of vanadium consumption is further complicated by the existence of multiple oxidation states with the potential to interconvert via oxidation-reduction, both in the environment and following ingestion. Highly oxidized forms of vanadium, +3 to +5, are considered the most stable forms and are most likely to be found as environmental contaminants; vanadyl sulfate, a tetravalent form (VIV ), is one of the dietary supplements advertised for diabetes and cholesterol. Studies in the literature suggest that oral exposure to vanadium (VIV or VV ) may have immunological, neurological, reproductive/developmental and general toxicity effects [12] , [6] , [7] , [11] , [21] , [24] and [25] . Despite the numerous studies published on vanadium compounds, the available literature lacks an adequate comparison of VIV and VV , under a standard study design, in two rodent species exposed in drinking water, the most relevant route of exposure.
Vanadium has gained recognition on the federal level by being included in the U.S. Environmental Protection Agency’s (U.S. EPA) draft drinking water Contaminant Candidate List 4, or CCL4, which will be finalized in 2016 [29] . Tetravalent and pentavalent vanadium compounds were nominated to the NTP for testing, in part by the U.S. EPA, and the critical data gaps have been outlined by the Agency for Toxic Substances and Disease Registry [2] . Some data gaps highlighted by ATSDR include comprehensive subchronic and chronic toxicity testing, with an adequate comparison of different oxidation states under similar testing conditions. To help address uncertainties regarding the safety of oral exposure to vanadium, the NTP conducted 14-day drinking water studies of vanadyl sulfate (VIV ) and sodium metavanadate (VV ) in adult Harlan Sprague Dawley rats and B6C3F1/N mice at exposure concentrations of 0, 125, 250, 500, 1000 or 2000 mg/L. These exposure concentrations were selected based on a critical review of the literature and consideration of the following: palatability, adult versus perinatal toxicity, the relative toxicity of pentavalent versus tetravalent compounds and an overall lack of toxicity data in mice. Vanadyl sulfate was selected as the VIV test article due to its use in dietary supplements. Sodium metavanadate was selected as a representative VV species following characterization of drinking water formulations of sodium orthovanadate and sodium metavanadate [13] . The designs of the two studies were essentially identical to facilitate comparisons between the two valence states. These data will help inform the design and exposure concentration selection for subchronic and chronic toxicity assessments of vanadium, which will provide essential data for the risk assessment of vanadium following oral exposure.
2. Methods
2.1. Chemical and dose formulation
Vanadyl sulfate (CASRN 27774-13-6, Lot No.0210324/1.1) was purchased from Noah Technologies Corporation (San Antonio, TX). Sodium metavanadate (CASRN 13718-26-8, Lot No. 8579K) was purchased from MP Biomedicals (Santa Ana, CA). The identity of each vanadium compound was determined by infrared spectroscopy, X-ray diffraction, and proton-induced X-ray emission spectroscopy. The overall purity, determined by high performance liquid chromatography (HPLC) with charged aerosol detection, was 100 and 99.5% for vanadyl sulfate and sodium metavanadate, respectively.
Drinking water formulations (0, 125, 250, 500, 1000, 2000 mg/L) of sodium metavanadate (∼pH 7) and vanadyl sulfate (∼pH 3.5) were prepared using tap water from the City of Columbus, Ohio municipal supply. Formulations were analyzed with HPLC with ultraviolet detection prior to study start and following the last exposure; all samples were within ±10% of the target concentration and 0 mg/L formulations were below the limit of quantitation of the analytical method (vanadyl sulfate 0.43 mg/L, sodium metavanadate 0.46 mg/L).
The stability of formulations was established prior to study start. Sodium metavanadate and vanadyl sulfate formulations were stable for up to 42 days; at day 42, sodium metavanadate was 99–101% of day 0 and vanadyl sulfate was 92–100% of day 0 when stored protected from light at ∼5 °C or room temperature. In some vanadyl sulfate formulations, a small peak was observed ≥day 7, in the HPLC chromatogram at the retention time of vanadate, suggesting slight oxidation of vanadyl to vanadate [13] .
2.2. Animals and animal maintenance
The studies presented here were conducted at Battelle Memorial Institute (West Jefferson, OH). Harlan Sprague Dawley (HSD) rats were purchased from Harlan (Indianapolis, IN). B6C3F1/N mice were supplied from the NTP colony at Taconic Biosciences (Germantown, NY). Upon arrival, animals were quarantined for at least 10 days and provided ad libitum access to irradiated NTP-2000 wafer feed (Zeigler Brothers, Inc., Gardeners, PA) and tap water. Following quarantine, 5–7 week-old animals were randomized into exposure groups by body weight. Animals were uniquely identified by tail tattoo upon randomization. On study day one, animals were placed on control or dosed-water (125, 250, 500, 1000, 2000 mg/L) ad libitum . Rats and female mice were housed 5 animals per cage and male mice were individually housed.
Animal use was in accordance with the U.S. Public Health Service policy on humane care and use of laboratory animals and the Guide for the Care and Use of Laboratory Animals[14] . Animals were treated humanely and with regard for alleviation of suffering. These studies were conducted in compliance with the Food and Drug Administration Good Laboratory Practice Regulation [8] .
2.3. Study design
Groups of 5 male and 5 female HSD rats and B6C3F1/N mice were exposed to vanadyl sulfate or sodium metavanadate at concentrations of 0, 125, 250, 500, 1000 or 2000 mg/L for 2 weeks. Water consumption was measured twice weekly. Animals exposed to sodium metavanadate at concentrations of 1000 and 2000 mg/L were removed from the study by day 9; therefore, values in Tables 2A and B and 3 represent the average for the first week only for those groups. Animals were observed for mortality and morbidity twice daily. Body weights were recorded prior to study start and twice weekly thereafter. All animals were euthanized humanely by CO2 inhalation and subjected to complete necropsies and gross examination of tissues. Organ weights for the liver, thymus, kidneys, testes, epididymides, ovaries, heart and lungs were recorded; all bilateral organs were weighed separately.
2.4. Statistical methods
Body weights, organ weights and water consumption were analyzed for treatment related effects by the Jonchkeere trend test. Pairwise comparisons were done using either the Shirley or Dunn tests for water consumption and the Williams or Dunnett tests for body and organ weights.
3. Results
3.1. Survival and clinical observations
Exposure to vanadyl sulfate had no impact on survival in male or female, HSD rats or B6C3F1/N mice. Male rats exposed to vanadyl sulfate exhibited thinness in the 2000 mg/L exposure group only, while female rats exhibited thinness at lower exposure concentrations and hunched posture at 2000 mg/L (Table 1 A). No clinical observations were noted in male rats below 2000 mg/L or female rats below 1000 mg/L. Clinical observations for mice exposed to high exposure concentrations of vanadyl sulfate included ruffled coat, hunched posture and thinness (Table 1 B). No clinical observations were noted for mice in the 125, 250 or 500 mg/L groups.
| (A) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | None | None | None | None |
| 125 | None | None | None | None |
| 250 | None | None | None | None |
| 500 | None | None | None | •Thin (4/5) |
| 1000 | •Thin (3/5) | •Thin (3/5) | None | •Thin (5/5) |
| •Ruffled coat (2/5) | ||||
| •Hunched posture (2/5) | ||||
| •Shallow breathing (1/5) | ||||
| •Lethargic (2/5) | •Lethargic (2/5) | |||
| •Abnormal gait (2/5) | ||||
| •Discharge | ||||
| Eye, brown (3/5) | ||||
| Nose, red (1/5) | ||||
| 2000 | •Thin (5/5) | •Thin (4/5) | •Thin (5/5) | •Thin (5/5) |
| •Ruffled coat (3/5) | •Ruffled coat (5/5) | |||
| •Hunched posture (2/5) | •Hunched posture (1/5) | •Hunched posture (2/5) | ||
| •Shallow breathing (1/5) | ||||
| •Lethargic (0/5) | •Lethargic (1/5) | |||
| •Abnormal gait (3/5) | •Abnormal gait (2/5) | |||
| •Discharge | •Discharge | |||
| Eye, brown (1/5) | Nose, red (1/5) | |||
| Nose, red (1/5) | ||||
| (B) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | None | None | None | None |
| 125 | None | None | None | None |
| 250 | None | None | None | None |
| 500 | •Thin (2/5) | •Thin (2/5) | None | None |
| •Ruffled coat (1/5) | •Ruffled coat (2/5) | |||
| •Hunched posture (1/5) | •Hunched posture (2/5) | |||
| •Shallow breathing (1/5) | ||||
| •Rapid Breathing (1/5) | ||||
| •Lethargic (1/5) | ||||
| •Abnormal gait (1/5) | •Abnormal gait (2/5) | |||
| 1000 | •Thin (4/5) | •Thin (5/5) | •Thin (2/5) | •Thin (5/5) |
| •Ruffled coat (5/5) | •Ruffled coat (5/5) | |||
| •Hunched posture (5/5) | •Hunched posture (4/5) | |||
| •Shallow breathing (4/5) | •Shallow breathing (1/5) | |||
| •Rapid breathing (1/5) | ||||
| •Lethargic (5/5) | ||||
| •Abnormal gait (5/5) | •Abnormal gait (5/5) | |||
| 2000 | •Thin (1/5) | •Thin (5/5) | •Thin (5/5) | •Thin (5/5) |
| •Ruffled coat (5/5) | •Ruffled coat (4/5) | •Ruffled coat (3/5) | •Ruffled coat (1/5) | |
| •Hunched posture (5/5) | •Hunched posture (5/5) | •Hunched posture (3/5) | ||
| •Shallow breathing (1/5) | ||||
| •Rapid Breathing (2/5) | ||||
| •Lethargic (1/5) | ||||
| •Abnormal gait (5/5) | •Abnormal gait (5/5) | |||
Decreased survival was observed in mice and rats exposed to high concentrations of sodium metavanadate. For rats, one male and one female in the 2000 mg/L group were found dead on days 6 and 7, respectively. In mice, three males and one female in the 1000 mg/L exposure group were found dead on days 7 and 8. No deaths were observed in rats in the 1000 mg/L group or mice in the 2000 mg/L groups, however, due to clinical observations, lower water consumption and mortality in other groups, the decision was made to remove all remaining rats and mice in the 1000 and 2000 mg/L groups by day 8. One male mouse in the 500 mg/L group was found dead on day 8, however mortality/moribunidty was not seen in other male mice in this group.
Additional clinical observations were noted in animals exposed to sodium metavanadate compared to vanadyl sulfate. Rats exposed to sodium metavanadate exhibited thinness, ruffled coat, hunched posture, shallow breathing, lethargy, abnormal gait and discharge from the eye (brown) and nose (red) (Table 1 A). These observations were noted in male rats at 1000 or 2000 mg/L, while female rats only displayed most of these symptoms at the highest exposure concentration. No clinical observations were noted in rats exposed to less than 1000 mg/L sodium metavanadate.
Overall, mice, especially females, appeared to be more sensitive to the effects of sodium metavanadate exposure compared to rats, with most clinical observations being noted down to the 500 mg/L exposure group (Table 1 B). Similar to the rats, mice exhibited thinness, ruffled coat, hunched posture, shallow breathing, rapid breathing, lethargy and abnormal gait. Only one male mouse in the 500 mg/L group, the animal found dead on day 8, exhibited clinical observations other than thinness. No clinical observations were noted in mice exposed to 125 or 250 mg/L sodium metavanadate.
3.2. Water and chemical consumption
Lower water consumption, likely due to palatability, was observed for both compounds, at nearly all concentrations in exposed animals. Animals in the 2000 mg/L exposure groups (vanadyl/vanadate, males/females, rats/mice) consumed between 67.6 and 86.5% less water than their concurrent controls (Table 2A and B ). Lower water consumption resulted in less than proportional compound exposure, at concentrations greater than 250 mg/L. Exposure to the compound and total vanadium, based on water consumption, is shown in Table 3 A (rats) and B (mice). Vanadium comprises 31% of vanadyl sulfate and 41.7% of sodium metavanadate as a weight percentage; therefore, animals exposed to sodium metavanadate consume more vanadium, per gram of water, than animals exposed to vanadyl sulfate, at the same exposure concentration. Due to early removal, consumption data presented in Tables 2A and B and 3 for 1000 or 2000 mg/L sodium metavanadate represent the average for study days 1–8 only.
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 22.0 | 17.0 | 27.9 | 18.0 |
| 125 | 21.0 (−4.5) | 17.0 (0.0) | 22.1 (−20.8)** | 17.2 (−4.4)** |
| 250 | 18.8 (−14.5) | 14.2 (−16.5) | 21.3 (−23.7)** | 16.3 (−9.4)** |
| 500 | 14.9 (−32.3) | 11.7 (−31.2) | 18.6 (−33.3)** | 14.4 (−20.0)** |
| 1000 | 6.5 (−70.5) | 7.2 (−57.6) | 14.6 (−47.7)** | 10.1 (−43.9)** |
| 2000 | 3.6 (−83.6) | 2.3 (−86.5) | 6.0 (−78.5)** | 4.1 (−77.2)** |
Values represent group averages (percent difference from 0 mg/L group). No standard error is presented because the animals were group housed 5 animals/cage and 5 animals/group. Averages for vanadyl sulfate and 0–500 mg/L sodium metavanadate are for study days 1–15. The averages presented for 1000 and 2000 mg/L sodium metavanadate are for study days 1–8, due to early removal.
- . Indicates statistical significance, p-value ≤ 0.01.
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 3.4±0.1 | 2.0 | 3.4±0.2 | 2.5 |
| 125 | 3.3±0.1 (−2.9) | 2.2 (10.0) | 3.3±0.0 (−2.9) | 2.1 (−16.0)** |
| 250 | 3.7±0.6 (1.1) | 1.9 (−5.0) | 3.3±0.1 (−2.9) | 1.7 (−32.0)** |
| 500 | 2.4±0.2 (−29.4)** | 1.2 (−40.0) | 2.5±0.1 (−26.5)** | 1.4 (−44.0)** |
| 1000 | 1.1±0.1 (−67.6)** | 0.7 (−65.0) | 1.8±0.1 (−47.1)** | 0.9 (−64.0)** |
| 2000 | 1.0±0.1 (−70.6)** | 0.3 (−85.0) | 1.1±0.1 (−67.6)** | 0.5 (−80.0)** |
Values represent group averages ± SEM (percent difference from 0 mg/L group). Standard error is only presented for male mice; female mice were group housed 5 animals/cage with 5 animals/group while male mice were singly housed. Averages for vanadyl sulfate and 0–500 mg/L sodium metavanadate are for study days 1–15. The averages presented for 1000 and 2000 mg/L sodium metavanadate are for study days 1–8, due to early removal.
- . Indicates statistical significance, p-value ≤ 0.01.
| (A) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| 125 | 12.9 (5.4) | 14.5 (6.0) | 13.1 (4.1) | 15.2 (4.7) |
| 250 | 24.1 (10.4) | 25.3 (10.5) | 26.0 (8.1) | 29.1 (9.0) |
| 500 | 39.1 (16.3) | 42.5 (17.7) | 46.2 (14.3) | 51.7 (16.0) |
| 1000 | 43.4 (18.1) | 59.6 (24.8) | 76.2 (23.6) | 75.6 (23.5) |
| 2000 | 48.2 (20.1) | 43.8 (18.3) | 77.5 (24.0) | 80.1 (24.8) |
| (B) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| 125 | 18.4 (7.7) | 15.3 (6.4) | 17.4 (5.4) | 14.9 (4.6) |
| 250 | 41.1 (17.1) | 26.5 (11.0) | 34.6 (10.7) | 25.3 (7.8) |
| 500 | 58.2 (24.3) | 38.5 (16.1) | 53.1 (16.5) | 42.4 (13.1) |
| 1000 | 60.3 (25.1) | 48.8 (20.3) | 81.3 (25.2) | 60.7 (18.8) |
| 2000 | 112.5 (46.9) | 46.7 (19.5) | 115.2 (35.7) | 83.6 (25.9) |
Values represent group averages for compound consumption and (total vanadium). Vanadium consumption was calculated from water consumption/chemical consumption; vanadium comprises 31% of vanadyl sulfate and 41.7% of sodium metavanadate. Averages for vanadyl sulfate and 0–500 mg/L sodium metavanadate are for study days 1–15. The averages presented for 1000 and 2000 mg/L sodium metavanadate are for study days 1–8, due to early removal.
3.3. Body weights
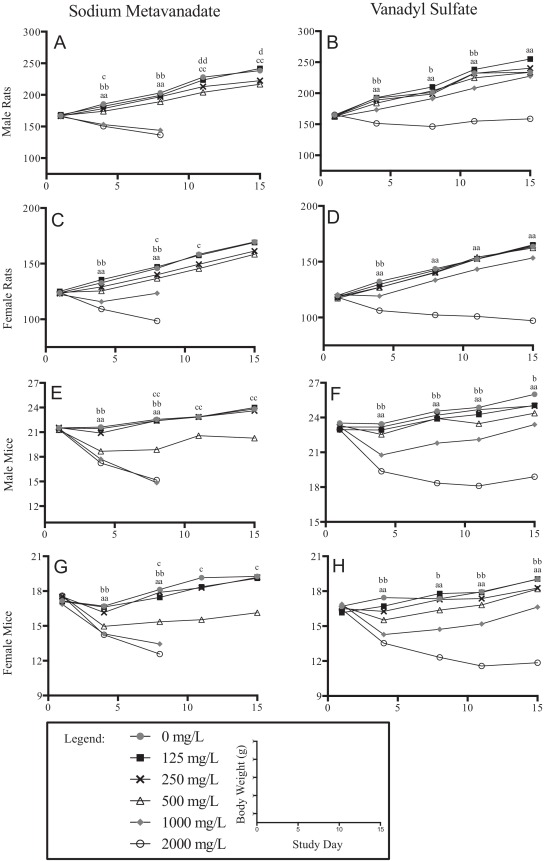
Lower body weights, compared to controls, and sometimes decreases in body weight, were observed in both sex/species exposed to either vanadyl sulfate or sodium metavanadate in the 2000 mg/L groups (Fig. 1 ). Lower water consumption likely contributed to body weight effects seen in the 2000 mg/L exposure groups. Rats exposed to vanadyl sulfate had significantly lower body weights at study termination in the 2000 mg/L group only (Fig. 1 B, D), while mice exposed to vanadyl sulfate had lower body weights in the 1000 and 2000 mg/L groups at termination (Fig. 1 F, H).
|
|
|
Fig. 1. Body Weight Curves. Body weight curves for animals exposed to sodium metavanadate are found in the left column and vanadyl sulfate in the right column, allowing comparison between compounds within a given sex/species; males rats are the first row (A, B), female rats the second row (C, D), male mice the third row (E, F) and female mice the fourth row (G, H). The x-axes represent study day and the y-axes are body weight in grams. Values shown are group averages, n = 4–5. Standard errors are not shown to increase visibility of the curves; all standard errors were less than 10% and often less than 5%. Statistical significance is shown for pair-wise tests against concurrent controls, single letter is p-value ≤ 0.05, double letter is p-value ≤ 0.01: ‘a’ for 2000 mg/L vs. 0/mg/L, ‘b’ for 1000 mg/L vs. 0 mg/L, ‘c’ for 500 mg/L vs. 0 mg/L, ‘d’ for 250 mg/L vs. 0 mg/L. Legend: closed grey circles are used for 0 mg/L, closed black squares are used for 125 mg/L, x marks are used for 250 mg/L, open black triangles are used for 500 mg/L, closed grey diamonds are used for 1000 mg/L and open black circles are used for 2000 mg/L. |
Despite similarities in water/compound consumption between animals exposed to vanadyl sulfate or sodium metavanadate, sodium metavanadate-exposed animals experienced effects on body weight at lower doses, down to 250 mg/L for male rats (Fig. 1 A). Body weight effects observed early for female rats were resolved at study termination in surviving groups (Fig. 1 C). Male and female mice had body weight effects in the 500 mg/L group at study termination and in the 1000 and 2000 mg/L groups prior to early removal (Fig. 1 E, G).
3.4. Organ weights
Significant organ weight changes were observed in several tissues, however most changes were likely related to body weight effects. Organ weights for sodium metavanadate are only available for the groups that were euthanized at study termination (day 15): 0, 125, 250 and 500 mg/L. Male rats, only, had significantly higher liver weights following exposure to both compounds (Table 4 ). The dose response following exposure to vanadyl sulfate was not strong, showing higher weights, compared to controls, at 125, 250 and 1000 mg/L but not at 500 mg/L; body weight effects at 2000 mg/L resulted in a lower liver weight compared to controls. Male rats exposed to sodium metavanadate had higher liver weights at 125 and 250 mg/L, however not at 500 mg/L, despite body weights being within 10% of controls.
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
|---|---|---|---|---|
| Liver | Kidney (L)a | Liver | Kidney (L)a | |
| 0 | 8.05±0.29 | 0.82±0.03 | 7.51±0.23 | 0.83±0.01 |
| 125 | 9.50±0.19** (17.98) | 0.80±0.03 (−2.44) | 10.12±0.22** (34.75) | 0.90±0.02 (8.43) |
| 250 | 9.45±0.44** (17.41) | 0.91±0.02** (10.98) | 10.24±0.28** (36.35) | 0.95±0.03 (14.46) |
| 500 | 8.42±0.24** (4.57) | 0.82±0.03 (0.00) | 7.42±0.21 (−1.20) | 0.85±0.02 (2.41) |
| 1000 | 10.75±0.45** (43.14) | 1.02±0.03** (22.89) | ||
| 2000 | 6.46±0.54** (−13.98) | 0.70±0.04** (−15.66) | ||
Organ weights recorded at necropsy. Values represent group averages ± SEM, n = 4–5. Value in parenthesis is the percent different from controls.
a. Organ weights shown are for the left (L) kidney.
- . Indicates statistical significance, p-value ≤ 0.01.
Kidney weights followed a similar trend as liver weights in male rats exposed to vanadyl sulfate (Table 4 ); higher kidney weights were seen at 1000 mg/L, while body weight effects resulted in lower kidney weights at 2000 mg/L. Increased kidney weights were only observed in male rats exposed to 250 mg/L sodium metavanadate with no significant difference observed in the 500 mg/L exposure group. Increased liver or kidney weights were not observed in any other sex/species exposed to either compound.
Lung weights in male and female mice exposed to sodium metavanadate were significantly lower than controls in all exposed groups in the absence of body weight effects, ranging from −30 to −40% compared to controls (Table 5 B). Lower lung weights, that did not reach significance, were also observed in other groups in the absence of body weight effects, e.g. male rats exposed to vanadyl sulfate or sodium metavanadate (Table 5 A). Effects on the thymus were observed in all animals exposed to vanadyl sulfate, usually in the presence of smaller magnitude body weight effects and likely stress related (Table 6 ). Mice exposed to sodium metavanadate also had lower thymus weights (males: −50.0%, females: −28.6% at 500 mg/L) in the presence of smaller magnitude body weight effects (data not shown).
| (A) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 2.36±0.36 | 1.33±0.08 | 2.11±0.18 | 1.37±0.04 |
| 125 | 1.90±0.13 (−19.49) | 1.40±0.06 (5.26) | 1.75±0.13 (−17.06) | 1.25±0.07 (−8.76) |
| 250 | 1.33±0.03** (−43.64) | 1.19±0.03 (−10.53) | 1.82±0.32 (−13.74) | 1.35±0.1 (−1.46) |
| 500 | 1.57±0.12 (−33.47) | 1.13±0.07 (−15.04) | 1.72±0.18 (−18.48) | 1.28±0.06 (−6.57) |
| 1000 | 1.37±0.09* (−35.07) | 1.13±0.05 (−17.52) | ||
| 2000 | 1.15±0.03 (−45.50) | 0.86±0.09 (−37.23) | ||
| (B) | ||||
|---|---|---|---|---|
| mg/L | Sodium Metavanadate | Vanadyl Sulfate | ||
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 0.28±0.02 | 0.23±0.01 | 0.21±0.02 | 0.16±0.01 |
| 125 | 0.17±0.01** (−39.28) | 0.16±0.01** (−30.43) | 0.18±0.01 (−14.29) | 0.16±0.01 (0.00) |
| 250 | 0.18±0.01** (−35.71) | 0.16±0.01** (−30.43) | 0.17±0.01 (−19.05) | 0.16±0.01 (0.00) |
| 500 | 0.16±0.01** (−42.86) | 0.14±0.01** (−39.13) | 0.19 ±0.01 (−9.52) | 0.16±0.01 (0.00) |
| 1000 | 0.18±0.01 (−14.29) | 0.14±0.01 (−12.50) | ||
| 2000 | 0.16±0.01 (−23.81) | 0.13±0.00** (−18.75) | ||
Organ weights recorded at necropsy. Values represent group averages ± SEM, n = 4-5. Value in parenthesis is the percent different from controls.
- . Indicates statistical significance, p-value ≤ 0.05.
- . Indicates statistical significance, p-value ≤ 0.01.
| mg/L | Rats | Mice | ||
|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | |
| 0 | 0.57±0.05 | 0.45±0.01 | 0.05±0.00 | 0.08±0.00 |
| 125 | 0.61±0.02 (7.02) | 0.42±0.03 (−6.67) | 0.05±0.00 (0.00) | 0.08±0.00 (0.32) |
| 250 | 0.54±0.03 (−5.26) | 0.45±0.02 (0.00) | 0.05±0.00 (0.00) | 0.07±0.00 (−12.5) |
| 500 | 0.49±0.02 (−14.04) | 0.41±0.03 (−8.89) | 0.05±0.00 (0.00) | 0.08±0.00 (0.00) |
| 1000 | 0.52±0.04 (−8.77) | 0.47±0.02 (4.44) | 0.06±0.01 (20.00) | 0.06±0.01 (−25.00) |
| 2000 | 0.36±0.04 (−36.84) | 0.25±0.09 (−44.44) | 0.02±0.00** (−60.00) | 0.02±0.00** (−75.00) |
Organ weights recorded at necropsy. Values represent group averages ± SEM, n = 4–5. Value in parenthesis is the percent different from controls.
- . Indicates statistical significance, p-value ≤ 0.01.
4. Discussion
The NTP has compared the short-term toxicity of vanadyl sulfate (VIV ) and sodium metavanadate (VV ) in HSD rats and B6C3F1/N mice following exposure via drinking water. Vanadium compounds, of various oxidation states, are of human health concern following oral exposure and are of interest to the U.S. Environmental Protection Agency for potential regulation in drinking water [29] .
Under the condition of these studies, it appears that sodium metavanadate was more toxic than vanadyl sulfate. While drinking water exposure to vanadyl sulfate or sodium metavanadate for 14 days resulted in similar levels of vanadium exposure (mg/kg/day) (Table 3 ), animals exposed to vanadate exhibited mortality/moribunidty and clinical signs of toxicity, e.g. lethargy, abnormal gait and breathing, which were not observed in vanadyl-exposed animals. The NTP has previously studied chromium compounds of different oxidation states and observed that the more oxidized form, a hexavalent compound, sodium dichromate dihydrate, was more toxic than the trivalent species, chromium picolinate monohydrate [17] , [18] , [26] and [27] .
One confounding variable in the interpretation of data in the present study is the reduced water consumption and potential dehydration of the animals. While some clinical observations noted are consistent with dehydration, the studies were not designed to directly assess dehydration. Further, it is unlikely that all observed effects were caused by dehydration. Lower water consumption was observed for all compounds, sexes and species; however, mortality and selected clinical observations were only noted in sodium metavanadate animals. For the 1000 and 2000 mg/L sodium metavanadate groups, it is possible that lower water consumption, combined with higher toxicity versus vanadyl sulfate, contributed to these effects. While the present studies indicate a difference in toxicity between vanadyl sulfate (VIV ) and sodium metavanadate (VV ), further studies are needed to gain a better understanding of potential differences in disposition and toxicity.
Higher organ weights observed in the present study may indicate that the liver and kidney are potential target organs following oral exposure to soluble vanadium compounds. Weight change in the liver of male rats was observed at the lowest exposure concentration for both compounds; however this weight change was not observed in any other sex/species. Studies in the literature suggest that vanadyl sulfate and sodium metavanadate may have effects on metabolic pathways, however no histopathological evidence of toxicity was observed in multiple studies with exposures up to one year at comparable doses to those used in the present studies [1] , [31] and [6] . Kidney weight changes were less consistent than liver changes in the male rats, however these changes may indicate potential toxicity. The literature available for kidney effects is variable; histopathologic changes have been reported in the kidney of rats exposed to sodium metavanadate for 3 months at 3.5 mg V/kg/day, while another study exposing at 19 mg V/kg/day for one year reported no histopathological lesions [31] and [6] .
Lower lung weights observed in the present study were fairly consistent between compounds and for most sex/species. These changes were, in most cases, in the absence of body weight effects. Data in the literature regarding the effect of vanadium exposure on the lung are limited to weight increases following inhalation exposure, e.g. [16] . Lower lung weight that is not associated with lower body weight is a novel finding with unknown toxicological relevance and will be considered further in subsequent, more comprehensive evaluations of vanadium toxicity following oral exposure.
Several studies in the literature, have reported effects of vanadium on developmental and reproductive endpoints following oral exposure [7] , [10] , [11] and [21] . In particular, decreased reproductive performance of male rats and mice (mated with naïve females), was reported following oral exposure to vanadyl sulfate or sodium metavanadate, respectively, for 8–9 weeks [10] and [11] . Weight changes in male reproductive-associated organs were not observed in the current studies, however more comprehensive assessment of effects on spermatogenesis would be needed to confirm whether or not vanadium has an impact on male reproductive performance.
The present studies will be used to select doses for subchronic toxicity evaluations of tetra- and pentavalent vanadium compounds in order to fill critical data gaps in the literature and help understand differences in toxicity between soluble vanadium with different oxidation states. Inter-conversion of oxidation state, in vivo, may complicate the interpretation of differences in toxicity, in these and future studies of vanadyl and vanadate compounds. The gastrointestinal (GI) environment has a wide range of pHs, resulting in ingested material experiencing both reduction and oxidation in different compartments of the GI tract following consumption. Oxidation, or more likely reduction reactions in the stomach, must also compete with gastric emptying to determine what species are present within lower sections of the GI tract. As a result, it is difficult to identify the oxidation state(s) of vanadium that are absorbed by the GI tract and distributed to tissues. In the NTP studies of hexavalent and trivalent chromium, oxidation state within tissues was not determined, however, tissue burden data of total chromium suggested that overall absorption of the hexavalent species was higher than the trivalent, helping to explain differences observed in toxicity [4] . Therefore, some understanding of total body burden may be used as a surrogate for determination of oxidation state in tissues and can help explain differences in toxicity. Studies in the literature have assessed the oral bioavailability of various vanadium compounds in tissues and urine, under different conditions, and reported a wide range of values from less than 1%, up to 16% [1] , [3] and [23] . By testing tetravalent and pentavalent vanadium under nearly identical conditions, the present and future NTP studies can begin to explore similarities or differences in biological response between the different oxidation states. As the NTP moves forward with subchronic evaluations of vanadyl sulfate and sodium metavanadate, it will be important to be aware of the potential for in vivo oxidation/reduction and to use available tools to assist in the explanation of differences in toxicity.
5. Conclusions
Drinking water exposure (0, 125, 250, 500, 1000, 2000 mg/L) of HSD rats and B6C3F1/N mice to vanadyl sulfate or sodium metavanadate (0, 125, 250, 500, 1000 or 2000 mg/L) resulted in lower water consumption at most doses with accompanying decreases in body weight at the highest exposure concentrations. Clinical observations and toxicity observed suggest that sodium metavanadate is more toxic than vanadyl sulfate. Despite having similar consumption of compound and total vanadium between vanadyl and vanadate-exposed animals, rats and mice in vanadate exposure groups exhibited lethargy, abnormal gait and abnormal breathing that were not observed in animals following vanadyl sulfate exposure. Based on these studies, the NTP has designed subchronic drinking water studies of vanadyl sulfate and sodium metavanadate in HSD rats, following perinatal exposure, and B6C3F1/N mice at concentrations of 0, 31.3, 62.5, 125, 250 or 500 mg/L for a more comprehensive comparison and assessment of toxicological characterization. These studies will include an extensive histopathological evaluation in rats and mice, as well as additional assessments in rats, including developmental landmarks, absorption/excretion and red cell/plasma partitioning of vanadium.
Transparency document
Transparency Document.
Acknowledgments
This work was performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S Department of Health and Human Services, under contract No HHSN273201400015C. Prior to submission this work was internally reviewed by Drs. Scott Masten and Cynthia Rider.
References
- [1] A. Adachi, K. Ogawa, Y. Tsushi, N. Nagao, T. Okano; Balance, excretion and tissue distribution of vanadium in rats after short-term ingestion; J. Health Sci., 46 (1) (2000), pp. 59–62
- [2] Agency for Toxic Substances and Disease Registry (ATSDR); A Toxicological Profile for Vanadium; U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta, GA (2012)
- [3] J. Azay, J. Bres, M. Krosniak, P.L. Teissedre, J.C. Cabanis, J.J. Serrano, G. Cros; Vanadium pharmacokinetics and oral bioavailability upon single-dose administration of vanadyl sulfate to rats; Fund. Clin. Pharmacol., 15 (2001), pp. 313–324
- [4] B.J. Collins, M.D. Stout, K.E. Levine, G.E. Kissling, R.L. Melnick, T.R. Fennell, R. Walden, K. Adbo, J.B. Pritchard, R.A. Fernando, L.T. Burka, M.J. Hooth; Exposure to hexavalent chromium resulted in significantly higher tissue chromium burden compared to trivalent chromium following similar oral doses to male F344/N rats and female B6C3F1 mice; Toxicol. Sci., 118 (2) (2010), pp. 368–379
- [5] D.G. Barceloux, D. Barceloux; Vanadium; J. Toxicol. Clin. Toxicol., 37 (2) (1999), pp. 265–278
- [6] J.L. Domingo, J.M. Llobet, J.M. Tomas, J. Corbella; Short-term toxicity studies of vanadium in rats; J. Appl. Toxicol., 5 (6) (1985), pp. 418–421
- [7] J.L. Domingo, J.M. Paternain, J.M. Llobet, J. Corbella; Effects of vanadium on reproduction, gestation, parturition and lactation in rats upon oral administration; Life Sci., 39 (1986), pp. 819–824
- [8] Food and Drug Administration; Good laboratory practice regulations. Final rule. 21CFR 58; Fed. Reg., 52 (172) (1987), pp. 33768–33782
- [9] International Agency for Research on Cancer; IARC monographs on the evaluation of carcinogenic risks to humans; Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide, vol. 86, IARC (2006), pp. 227––292 (accessed 18.11.15). URL: http://monographs.iarc.fr/ENG/Monographs/vol86/mono86.pdf
- [10] G.C. Jain, H. Pareek, S. Sharma, M. Bhardwaj, B.S. Khajja; Reproductive toxicity of vanadyl sulphate in male rats; J. Health Sci., 53 (1) (2007), pp. 137–141
- [11] J.M. Llobet, M.T. Colomina, J.J. Sirvent, J.L. Domingo, J. Corbella; Reproductive toxicity evaluation of vanadium in male mice?; Toxicology, 80 (2–3) (1993), pp. 199–206
- [12] J.M. Llobet, J.L. Domingo; Acute toxicity of vanadium compounds in rats and mice; Toxicol. Lett., 23 (2) (1984), pp. 227–231
- [13] E. Mutlu, T. Cristy, S.W. Graves, M.J. Hooth, S. Waidyanatha; Characterization of aqueous formulations of tetra- and pentavalent forms of vanadium in support of toxicology studies; Environ. Sci. Pollut. (2016) (submitted)
- [14] National Research Council; Guide for the Care and Use of Laboratory Animals; National Academies Press, Washington, DC (1996)
- [15] NRC (National Research Council); Vanadium; Mineral Tolerance of Animals (2nd revised ed.), National Academy Press, Washington, DC (2005), pp. 398–412 (Chapter 30)
- [16] National Toxicology Program (NTP); Toxicology and Carcinogenesis Studies of Vanadium Pentoxide (CAS No. 1314-62-1) in F344/N Rats and B6C3F1 Mice (Inhalation Studies). Technical Report Series No. 507. NIH Publication No. 03-4441 ; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC (2000)
- [17] National Toxicology Program (NTP); Toxicology and Carcinogenesis Studies of Sodium Dichromate Dihydrate (CAS No. 7789-12-0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). Technical Report Series No. 546. NIH Publication No. 08-5887 ; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC (2008)
- [18] National Toxicology Program (NTP); Toxicology and Carcinogenesis Studies of Chromium Picolinate Monohydrate (CAS No. 27882-76-4) in F344/N Rats and B6C3F1 Mice (Feed Studies). Technical Report Series No. 556. NIH Publication No. 10-5897 ; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC (2010)
- [19] North Carolina Department of Environment and Natural Resources (NC DENR); Summary of Well Testing Near Coal Ash Ponds. 28 August; NC DENR (2015) (accessed 25.11.15). URL:. http://portal.ncdenr.org/c/document_library/get_file?uuid=09b93b4b-039f-4986-bc50-7d1227b422bb&groupId=14
- [20] Office of Environmental Health Hazard Assessment, Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. State of California Environmental Protection Agency. Office of Environmental Health Hazard Assessment, (accessed 07.12.15). URL: http://oehha.ca.gov/proposition-65/proposition-65-list
- [21] J.L. Paternain, J.L. Domingo, M. Gomez, A. Ortega, J. Corbella; Developmental toxicity of vanadium in mice after oral administration; J. Appl. Toxicol., 10 (3) (1990), pp. 181–186
- [22] D.E. Polyak; Vanadium. 2013 Minerals Yearbook; USGS (2007)
- [23] A.V. Rochin, E.K. Ordzhonikidze, I.V. Shalganova; Vanadium-toxicity, metabolism, carrier state; J. Hyg. Epidemiol. Microbiol. Immunol., 24 (1980), pp. 377–383
- [24] D. Sanchez, A. Oretga, J.L. Domingo, J. Corbella; Developmental toxicity evaluation of orthovanadate in the mouse; Biol. Trace Elem. Red., 30 (3) (1991), pp. 219–226
- [25] R.P. Sharma, D.R. Bourcier, C.R. Brinkerhoff, S.A. Christensen; Effects of vanadium on immunologic functions; Am. J. Ind. Med., 2 (2) (1981), pp. 91–99
- [26] M.D. Stout, R.A. Herbert, G.E. Kissling, B.J. Collins, G.S. Travlos, K.L. Witt, R.L. Melnick, K.M. Abdo, D.E. Malarkey, M.J. Hooth; Hexavalent chromium is carcinogenic to F334/N rats and B6C3F1 mice after chronic oral exposure; Environ. Health Perspect., 117 (5) (2009), pp. 716–722
- [27] M.D. Stout, A. Nyska, B.J. Collins, K.L. Witt, G.E. Kissling, D.E. Malarkey, M.J. Hooth; Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years; Food Chem. Toxicol., 47 (4) (2009), pp. 729–733
- [28] K.H. Thompson, C. Orvig; Vanadium in diabetes: 100 years from phase 0 to phase I; J. Inorg. Biochem., 100 (12) (2006), pp. 1925–1935
- [29] United States Environmental Protection Agency; Fact Sheet: Drinking Water Contaminant Candidate List 4-Draft; Office of Water, U.S. EPA (2015)
- [30] WHO; Vanadium. Air Quality Guidelines, Chapter 6.1; WHO (2000)
- [31] S. Dai, K.H. Thompson, E. Vera, et al.; Toxicity studies on one-year treatment of non-diabetic and streptozotocin-diabetic rats with vanadyl sulphate; Pharmacol. Toxicol., 5 (1994), pp. 265–273
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?