1. INTRODUCTION
Since the 1990s, efforts have been made to explore the benefit of using polymer nanocomposites to have reinforced polymers without a considerable increase in weight (1,2) This has been based on the idea that fillers with large surface area (at least one of their dimensions in nanometer size) have a high effectiveness since they maximize the particle-matrix contact, without the need to use large amounts of reinforcements, as long as there is good chemical compatibility, dispersion and distribution of the nanoparticle in the polymer matrix (3,4). Special interest has been taken in the use of polyolefin-based matrices, due to their price and versatility, especially in isostatic polypropylene (iPP) which is a material widely used in the automotive industry, construction, packaging, etc.
When iPP-based nanocomposites have been prepared, three major strategies have been followed: the simplest and most studied is based on melt intercalation with the use of compatibilizers (5–7), surface modifications on the nanoclay (8–10) or the application of an electric field to nanocomposites (11,12). All these efforts have been oriented to improve the chemical compatibility between the matrix and the filler and to avoid the formation of aggregates, since this significantly compromises the mechanical behaviour of the composites (13). The second strategy is oriented to prepare the nanocomposites in solution, in order to penetrate the layers or separate the aggregates from the nanoparticles more efficiently, introducing the dissolved polymeric chains between them, since they have greater mobility. This is a technique that even with very good results has barriers to commercialization (difficult to scale up, excessive use of solvent, etc.) (14,15). The last strategy is based on in-situ polymerisation, where the reinforcement is present from the synthesis process itself because it is part of the catalytic system, so that polymeric growth on the nanoparticles guarantees their compatibility and dispersion (13,16–19).
The results obtained in recent years in the in-situ polymerisation technique of iPP show that the modulus is significantly increased without compromising the stereospecificity of the molecule and significantly increases the molecular weights, resulting in materials with high stiffness. Its use makes it possible to obtain composites with a homogeneous distribution of reinforcement, in a wide range of compositions (20,21). However, although the surface area of these reinforcements is exploited more efficiently, the toughness of the material and the productivity of the reaction are significantly compromised with this technique, even more so in polyolefins as crystalline as iPP (22).There is practically no literature concerning in-situ polymerisation of sPP nanocomposites, although Polshchikov and co-workers (23) showed that in-situ polymerisation of sPP in the presence of carbon nanotubes did not lead to a significant decrease in the activity and stereospecificity of a syndiotactic catalyst. The mechanical behaviour of other types of sPP nanocomposites remains to be investigated.
In this work we studied for the first time the possibility of achieving a compromise of mechanical properties in PP matrix nanocomposites by supporting metallocene co-catalyst (MAO) in fibrillar nanoclay, which were used at the same time as reinforcement. Additionally, for addressing the problem of the low polarity of the polyolefinic chains of both PP configurations, polar monomers such as 1-undecenol (R-OH) and undecenoic acid (R-COOH) have been added, in low concentrations in the reaction medium, to study the possible synergies and the evolution of the microstructure of the different nanocomposites obtained.
2. EXPERIMENTAL
2.1. Materials
The polymerisation gas used was propene (supplied by Air Liquide). This gas is polymerisation grade, with less than 0.06 ppm of oxygen and 0.4 ppm of nitrogen. The following compounds were part of the catalyst system employed: dimethylsilylbisbenzyl zirconium (IV)-dichloride (Me2Si(Ind)2ZrCl2, Aldrich) and diphenylmethylidene (cyclopentadienyl) (fluorenyl) zirconium dichloride Ph2C(Flu)(Cp)ZrCl2, Aldrich); which were used as catalysts. Methylaluminoxane (MAO, 17 wt. % solution in toluene, AzkoNobel) or Trisobutylaluminum (TIBA, 25 wt. % solution in toluene, Sigma-Aldrich) were used as co-catalyst or cleaning agent, respectively. All were used without any treatment. The solvents (Toluene, Fisher Scientific) and comonomers (1-undecenol (R-OH) and undecenoic acid (R-COOH), Aldrich) used were previously distilled.
The clay used for the preparation of metallocene PP nanocomposites was a commercial sepiolite (SEP) supplied by TOLSA (unit fibre size 0.2–3 μm, 10–30 nm width, and 5–10 nm in thickness). These fillers were dried under vacuum at 80 °C for 24 h before treatment.
All materials sensitive to air, water and impurities were handled in an inert atmosphere under a flow of Nitrogen (99 % purity, Air Liquide) within a glovebox or inside the polymerisation reactor, respectively.
2.2. Clay treatment
The desired amount of dry clay was mixed with co-catalyst, in ratio 2 g clay/ml of MAO solution, in 100 ml of dry toluene and afterward the mixture was stirred for 90 min at room temperature, following the procedure explained in our previous works for optimizing the process of immobilization of the MAO in sepiolite (17,22,24). The resulting solid was washed three times with 30 ml of fresh toluene and dried inside globe box until it was used.
2.3. Polymerisation
Polymerisation of different neat polypropylenes (iPP, sPP and iPP-sPP) was carried out following a rigorous cleaning process of the reactor. The polymerisation temperature was set at 20 °C, once achieved, 0.05 M solution of TIBA in toluene was transferred to the reactor and kept under propene atmosphere stirring for 5 minutes at 600 rpm. In a second step, the reactor was fed with 3.0x10-6 mols of a stereo-specific catalyst in 100ml of toluene and the amount of MAO solution suitable to have an Al/Zr ratio of 1:1200 (In the case of polymerisation in the presence of polar comonomers this ratio was increased to 1:2000). The reaction started with the propene injection at 5 bars and held for one hour.
The catalyst mixture retained 3.0x10-6 total Zr moles in the polymerisation reactor.
The catalytic activity was stopped by hydrolysing the MAO with the addition of 100 ml of a mixture of ethanol and 10 %v of hydrochloric acid. The polymer was precipitated in 800 ml of water and kept under stirring for 12 hours. It was finally filtered and dried at 80ºC under vacuum for another 12 hours approximately.
All materials were polymerized with different initial amounts of treated nanoclay (0.5 y 1 g) to obtain their corresponding nanocomposites: N0.5iPP, N1sPP, N0.5sPP, N1sPP. These nanoparticles were added in the second stage of the described polymerisation protocol together with the corresponding catalyst.
In the case of nanocomposites with polar comonomers (R-OH or R-COOH), these were added together with the treated nanoclay and the stereospecific catalyst (C2 or CS symmetry) before feeding the polymerisation gas into the reactor. The amounts of monomer used were 3x10-3 or 7x10-3 [M]. In the study of the influence of polar comonomers, a full factorial design (FFD) with four factors at two levels of assessment was used. Table 1 shows the DoE model.
| Factor | Level -1 | Level +1 | |
| A | Catalyst type | C2 symmetry | Cs symmetry |
| B | Initial amount of nanoclay (g) | 0.25 g | 0.5 g |
| C | Comonomer type | R-OH | R-COOH |
| D | Polar comonomer concentration [M] | 3 x 10-3 | 7 x10-3 |
2.4 Sample preparation
The obtained nanocomposites were homogenised with two thermal stabilizers for polyolefins (Irganox 1010 and Irgafos 168 supplied by Ciba, Spain) during extrusion process at 210 °C/80 rpm. At a later stage were moulded in a Schwabenthan hot plate press, heating at 200 °C, for 5 minutes without pressure and the second step was applied 10 MPa with an additional 10 minutes. Finally, the plates were stamped with the specific dimensions for each characterization test.
2.5 Characterization
In a polymerisation process, the most important characteristic parameter is productivity. The productivity was measured as (kilograms of polyolefin)/(Zr moles x pressure x time).
The melt and crystallization temperatures (Tm, Tc), as well as the heat of fusion (ΔHm) and degree of crystallinity of samples (Xc), were measured by a Mettler Toledo DSC 821e thermal analysis system model (DSC) in the temperature range from 25 to 250 °C at a heating rate of 20 °C min-1 under nitrogen flow. The samples were first heated to 250 °C for 2 min to eliminate their thermal history and subsequently cooled to 25 °C. The second endotherm was recorded by heating 20 °C min-1, the X, Tm, and ΔHm of the samples were calculated, taking the melting heat of neat PP as 190 J/g (25). Thermogravimetric Analysis (TGA) was used to determine the clay content in the obtained nanocomposites. Thermograms were obtained in a nitrogen atmosphere with a heating rate of 20 °C min-1 using a Mettler Toledo TGA851e.
13C-NMR assays were used to determine the amount and distribution of the polar comonomers and the tacticity of the synthesized PP, through the identification of the couplings and molecular vibrations of the samples. 13C-NMR assays were performed on a 500 MHz NMR Spectrometer (BRUKER AVANCE III 500, 11.74 Teslas).
The mechanical properties of the resulting nanocomposites were measured as follows: Young’s modulus at a speed of 1 mm min-1 and tensile strength at a speed of 50 mm min-1 were measured according to UNE-EN ISO 527–1 and 527–2 with an Instron Model 5500R60025. In order to report specific properties, density was measured using an analytical balance Mettler Toledo, model AX205DR, under the standard ISO 1183-1.
3. RESULTS AND DISCUSSION
The incorporation of two types of polar comonomers (R-OH and R-COOH) in the syndiotactic and isotactic configuration has been studied, with the aim of improving the chemical compatibility between the matrix and the nanoclay in the nanocomposites. The polymerisation methodology followed is described in Figure 1. In order to focus on a significant number of factors and understand their influence on the final structure of the nanocomposite matrix, a full factorial design (FFD) and a computerised interaction interpretation tool (Minitab software) was used.
Figure 1. Scheme for obtaining iPP and sPP nanocomposites with polar comonomers
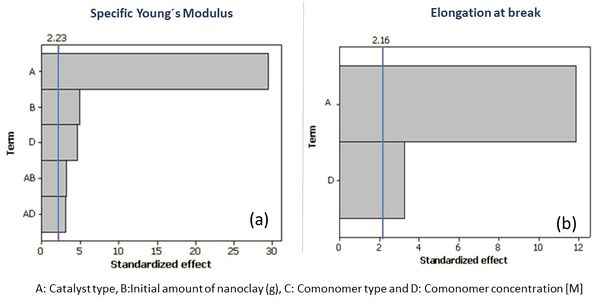
Table 2 presents the results of the two control properties studied, concerning the main mechanical properties (Specific Young's modulus and elongation at break), for the 16 experiments returned by the design of experiments used. Figure 2 represents the Pareto charts of the effects that allows to identify the most influential variables and to compare the relative magnitude of these effects.
| Run | Factors | Control properties | ||||
| Catalyst type
Factor-A |
Initial amount of nanoclay (g)
Factor-B |
Comonomer type
Factor-C |
Polar comonomer concentration [M]
Factor-D |
Specific Young´s Modulus (MPa.cm3.g-1) | Elongation at break (%) | |
| 1 | C2-symmetry | 0.25 | R-OH | 3x10-3 | 2156 ± 89 | 170 ± 12 |
| 2 | Cs-symmetry | 0.25 | R-OH | 3x10-3 | 1560 ± 120 | 301 ± 28 |
| 3 | C2-symmetry | 0.50 | R-OH | 3x10-3 | 2360 ± 108 | 120 ± 16 |
| 4 | Cs-symmetry | 0.50 | R-OH | 3x10-3 | 1612 ± 76 | 350 ± 22 |
| 5 | C2-symmetry | 0.25 | R-COOH | 3x10-3 | 2115 ± 52 | 108 ± 19 |
| 6 | Cs-symmetry | 0.25 | R-COOH | 3x10-3 | 1524 ± 165 | 417 ± 21 |
| 7 | C2-symmetry | 0.50 | R-COOH | 3x10-3 | 2264 ± 108 | 119± 11 |
| 8 | Cs-symmetry | 0.50 | R-COOH | 3x10-3 | 1598 ± 44 | 405 ± 32 |
| 9 | C2-symmetry | 0.25 | R-OH | 7x10-3 | 2120 ± 202 | 92 ± 15 |
| 10 | Cs-symmetry | 0.25 | R-OH | 7x10-3 | 1328 ± 81 | 298 ± 30 |
| 11 | C2-symmetry | 0.50 | R-OH | 7x10-3 | 2310 ± 64 | 98 ± 18 |
| 12 | Cs-symmetry | 0.50 | R-OH | 7x10-3 | 1452 ± 91 | 256 ± 14 |
| 13 | C2-symmetry | 0.25 | R-COOH | 7x10-3 | 2026 ± 69 | 117 ± 14 |
| 14 | Cs-symmetry | 0.25 | R-COOH | 7x10-3 | 1410 ± 102 | 306 ±28 |
| 15 | C2-symmetry | 0.50 | R-COOH | 7x10-3 | 2289 ± 77 | 90 ±17 |
| 16 | Cs-symmetry | 0.50 | R-COOH | 7x10-3 | 1325 ± 62 | 265 ± 33 |
Regarding the variables affecting Young's modulus, it is observed in Figure 2 (a) that in the analysis of variance (ANOVA), performed by the software, the linear terms: catalyst type (factor-A), initial amount of clay (factor-B) and comonomer concentration (factor-D), as well as the interaction catalyst type-initial amount of clay (factor-AB) and catalyst type-polar comonomer concentration (factor-AD), are statistically more significant on this property. Since the values are higher than the established P-statistic threshold (blue line on the curves). In the same way it is determined in Figure 2 (b) that only two linear terms: catalyst type (factor-A) and comonomer concentration (factor-D) are significant on the elongation at break.
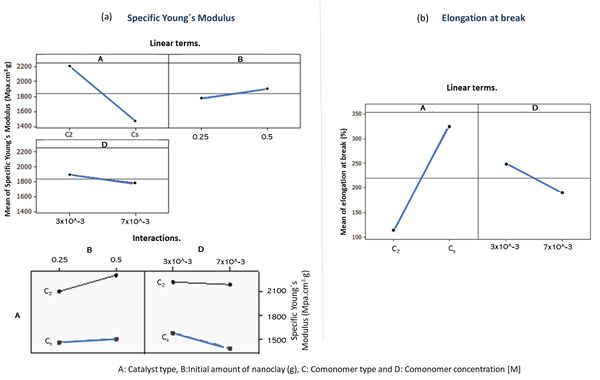
Once the most influential variables on the mechanical properties studied were identified, factorial plots were constructed (see Figure 3 (a) and (b)). Factorial plots are used to determine the impact of the factors on the responses. The slope of the line indicates the strength of the effect. Since the objective is to maximize both responses, it is clearly observed that the type of catalyst used (factor-A) is the most determinant in both measured properties. As expected, the influence is inverse: the isotactic catalyst (C2-symmentry) favours the high modulus and decreases the elongation at break.
The amount of initial nanoclay present in the polymerisation (factor-B), only significantly affects the specific Young's modulus, which is consistent with the results presented in the our previous work (26): at these low reinforcement percentages, there is an increase in tactility, crystallinity and molecular weight which favours stiffness (see Table 3). Additionally, increasing the factor-B increases the final amount of load and this fact, in turn, acts as a nucleating agent, which also explains the positive influence of the factor-B on the stiffness.
The non-significant effect of factor-B on elongation is also consistent with the results presented above: the amount of nanoclay, although increasing crystallinity and immobilising the polymer chains, but also it increases the syndiotactic sections, which could offset the effects, thus not playing a major role in toughness, as predicted by the model.
On the other hand, the type of polar comonomer (factor -C) was not decisive in any of the mechanical properties studied. In previous studies, it has been determined that the length of the comonomer plays an important because longer branches can increase the angle with the main chain, leaving space to continue with the addition of other monomers to the active complex (16,27). In this study, R:10 carbons were maintained for both (R-OH and R-COOH) in order to analyse only the compatibility and reactivity of the chemical group (hydroxide or acid). Although on equal conditions, it is shown the -OH group was more reactive (higher insertion measured by 13C-NMR, see table 3), neither of them showed a prominent role in the matrix-nanoclay compatibility as there was no higher deformation, nor higher stress transmission in the tensile tests.
Table 3. Microstructural characteristics of the obtained samples.
| Run | Microstructural characteristics | ||||||
| Productivity kg PP/mol Zr . h . bar. | Xc (%) | Mw (g/mol) | PI | Stereospecificity
mmmm or rrrr |
Final nanoclay (%) | Polar Comonomer (%) | |
| 1 | 5,10E+03 | 76 | 1.50E+05 | 2.7 | 87.6 | 0.8 | 0.64 |
| 2 | 4.30E+03 | 38 | 5.60E+05 | 3.7 | 75.8 | 3.1 | 0.48 |
| 3 | 4.00E+03 | 70 | 1.90E+05 | 2.2 | 89.1 | 1.9 | 0.69 |
| 4 | 3.02E+03 | 36 | 6.10E+05 | 2.9 | 86.1 | 3.8 | 0.53 |
| 5 | 5.23E+03 | 72 | 1.75E+05 | 2.3 | 89.9 | 0.7 | 0.45 |
| 6 | 4.26E+03 | 42 | 5.87E+05 | 3.2 | 79.2 | 2.8 | 0.35 |
| 7 | 4.20E+03 | 68 | 2.10E+05 | 2.1 | 92.1 | 1.7 | 0.57 |
| 8 | 3.68E+03 | 39 | 6.35E+05 | 2.5 | 88.7 | 3.6 | 0.46 |
| 9 | 4.03E+03 | 72 | 1.10E+05 | 3.8 | 72.6 | 2.1 | 0.72 |
| 10 | 3.02E+03 | 30 | 4.25E+05 | 4.2 | 62.1 | 4.2 | 0.55 |
| 11 | 3.70E+03 | 65 | 1.26E+05 | 3.1 | 79.2 | 2.4 | 0.75 |
| 12 | 2.01E+03 | 28 | 4.72E+05 | 3.6 | 67.9 | 5.7 | 0.62 |
| 13 | 3.97E+03 | 70 | 1.13E+05 | 2.9 | 76.2 | 2.2 | 0.56 |
| 14 | 2.26E+03 | 26 | 4.58E+03 | 3.8 | 69.2 | 4.8 | 0.46 |
| 15 | 3.40E+03 | 68 | 1.32E+05 | 2.7 | 77.8 | 2.6 | 0.62 |
| 16 | 2.15E+03 | 24 | 4.90E+03 | 3.5 | 72.8 | 5.2 | 0.58 |
Although the type of polar comonomer (factor-C) was not decisive, the amount of polar comonomer used (factor-D) was. In this case both properties are affected in the same way: little amount of comonomer improves both the stiffness and toughness of the nanocomposites.
At this point it is important to note that, beside the factor -B, factor -D is also decisive for the productivity of the reaction, therefore low amounts were used (< 7x10-3 M). Additionally, an increase of the Al/Zr ratio had to be used to avoid premature termination reactions. However, as can be seen in Table 3, the productivity of these reactions is still much lower than those reported in the absence of polar co-monomers (26).
Important reasons why the inclusion of polar vinyl comonomers, in pure polyolefins, decreases the productivity of the synthesis are listed in current literature: the first point is the observed poisoning of the metal centre by strongly bound polar monomers, which can lead to the formation of metalalkyl chelates. A second problem is the elimination of beta-x, where metal-x species are potential by-products. Another problem arises from the mismatch of the energies of the monomers with the catalyst's boundary orbitals. In all cases, this results in catalytically inactive species (28,29). These phenomena could be occurring in the synthesis of nanocomposites; hence the factor -D is decisive in productivity and final nanoclay. The increase in the amount of final reinforcement may also be the reason why the factor -D is decisive for both mechanical properties as it acts as an important nucleation agent, and at the same time, decreases the nanoclay-matrix polarity differences.
Although the model predicts that only the linear terms were decisive in both properties, in the case of the specific Young´s modulus, some secondary interactions also contribute. Figure 3 (a) plots this interaction of factors. These plots are used to visualize the interaction effect of factors on the responses and to compare the relative strength of effects. The greater the deviation from parallel, greater is the interaction between the factors. As expected, the greatest interplay occurs between the factors A-B, and maximum stiffness is achieved when the C2-symmetry catalyst and higher amount of nanoclay are used.The effects of the significant linear terms and their interactions are represented in equation 1 and 2. Although the statistical programme is able to describe linear regression equations that predict the mechanical behaviour of the nanocomposites, these equations use a non-quantitative factor (type of catalyst: factor -A) that does not allow them to be strictly used. However, the equations allow identifying the sensitivity of the mechanical properties studied to two common linear terms: the type of catalyst and the amount of polar comonomer used.
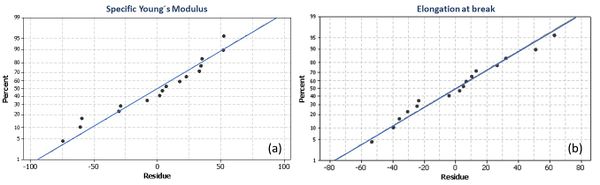
The residual plots presented in Figure 4 (a) and (b), show that the applied model is adequate and meets the assumptions of the analysis. Furthermore, the R2 value shows that the model explains 98.94% and 92.10% of the variance in the specific Young's modulus and elongation at break respectively, indicating that the model explains very well variations in the responses.
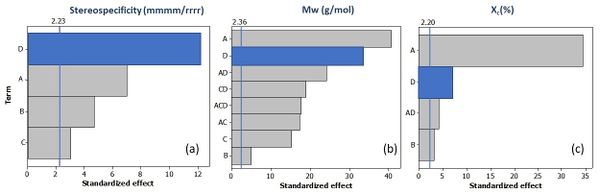
Considering the important impact of polar comonomers on mechanical properties, Pareto chart of normalised effects were analysed for properties such as stereoregularity, Mw and crystallinity. The results, presented in Figure 5 (a), (b) and (c) respectively, showed that the addition of the polar comonomer is one of the main determining factors in all these properties.
In the case of Mw, it can be seen that polar comonomers act chain transfer agent (such as hydrogen) to control Mw and polydispersity of obtained nanocomposites (increases Mw and narrows the distribution of molecular weights). The molecular mobility resulting from this loss of molecular weight could be a determining factor in the increase of crystallinity. In turn, the coordination mechanism of the catalysts is being affected by the polarity and volume (steric hindrance) of these comonomers. This chain of effects could explain the importance of the polar comonomer in the mechanical properties.
Finally, the results of the characterisation, shown in Table 2, also demonstrate that increasing the amount of nanoclay in the reaction medium contributes to improve the incorporation rate of the polar comonomer (provided that small amounts are used). This could also be due to the immobilisation of the catalyst on the support, as other authors have shown when incorporating alpha olefin monomers by in-situ polymerisation (16,30,31).
These studies demonstrate that there is a window of opportunity to improve the incorporation of functional monomers in coordination reactions through nano supported metallocene catalyst. Moreover, the presence of polar comonomers is determinant for modelling the microstructure of the nanocomposites obtained in the in-situ polymerization, independently of the stereospecificity of the catalyst used.
4. CONCLUSION
The interpretation of the full factorial design allowed us to conclude that the matrix configuration (iso or syndiotactic) is determinant in modelling the mechanical properties of polypropylene/clay nanocomposites. The use of polar comonomers, regardless of the chemical group used -OH or -COOH, was instrumental in simultaneously increasing the stiffness and toughness of the nanocomposites. Few significant interactions were found between the other factors studied.
Finally, besides demonstrating that the inclusion of polar comonomers significantly improves the mechanical properties of the obtained nanocomposites, it was also shown that in situ polymerisation increases the amount of polar comonomer insertion in the hydrocarbon chains of the synthesised nanocomposite.
Acknowledgements
The authors acknowledge the financial support of University of Valladolid for the UVA Postdoctoral Contract CONVOCATORIA 2020 (K.C.N.C).
References
1. Hasegawa N, Kawasumi M, Kato M, Usuki A, Okada A. Preparation and Mechanical Properties of Polypropylene - Clay Hybrids Using a Maleic. Macromolecules. 1997;30(20):6333–8.
2. Arimitsu Usuki, Yoshitsugu Kojima, Masaya Kawasumi, Akane Okada, Yoshiaki Fukushima TK& OK. Synthesis of nylon 6-clay hybrid. J Mater Res. 1993;8:1179–84.
3. Shameem MM, Sasikanth SM, Annamalai R, Raman RG. A brief review on polymer nanocomposites and its applications. Mater Today Proc. 2021;45:2536–9.
4. Tsioptsias C, Leontiadis K, Tzimpilis E, Tsivintzelis I. Polypropylene nanocomposite fibers: A review of current trends and new developments. J Plast Film Sheeting. 2020;0(0):1–29.
5. Kim DH, Fasulo PD, Rodgers WR, Paul DR. Effect of the ratio of maleated polypropylene to organoclay on the structure and properties of TPO-based nanocomposites. Part II: Thermal expansion behavior. Polymer (Guildf). 2008;49(10):2492–506.
6. Xu Y, Guo Z, Fang Z, Peng M, Shen L. Combination of double-modified clay and polypropylene-graft-maleic anhydride for the simultaneously improved thermal and mechanical properties of polypropylene. J Appl Polym Sci. 2013;128(1):283–91.
7. Lertwimolnun W, Vergnes B. Influence of compatibilizer and processing conditions on the dispersion of nanoclay in a polypropylene matrix. Polymer (Guildf). 2005;46(10):3462–71.
8. Gallego R, García-López D, Merino JC, Pastor JM. How do the shape of clay and type of modifier affect properties of polymer blends? J Appl Polym Sci. 2013;127(4):3009–16.
9. Papageorgiou GZ, Achilias DS, Bikiaris DN, Karayannidis GP. Crystallization kinetics and nucleation activity of filler in polypropylene/surface-treated SiO2 nanocomposites. Thermochim Acta. 2005;427(1–2):117–28.
10. Ren W, Jayaraman K. Blown films with balanced in-plane properties from polypropylene-clay nanocomposites though silane coupling. J Plast Film Sheeting. 2021;37(1):93–110.
11. Hyun K, Lim HT, Ahn KH. Nonlinear response of polypropylene (PP)/Clay nanocomposites under dynamic oscillatory shear flow. Korea Aust Rheol J. 2012;24(2):113–20.
12. Ock HG, Ahn KH, Lee SJ. Effect of electric field on polymer/clay nanocomposites depending on the affinities between the polymer and clay. J Appl Polym Sci. 2016;133(25):6–11.
13. Kim M, Song HY, Choi WJ, Hyun K. Evaluation of the Degree of Dispersion of Polymer Nanocomposites (PNCs) Using Nonlinear Rheological Properties by FT-Rheology. Macromolecules. 2019;52(22):8604–16.
14. Chiu FC, Chu PH. Characterization of solution-mixed polypropylene/clay nanocomposites without compatibilizers. J Polym Res. 2006;13(1):73–8.
15. Shi X, Gan Z. Preparation and characterization of poly(propylene carbonate)/montmorillonite nanocomposites by solution intercalation. Eur Polym J. 2007;43(12):4852–8.
16. Tanasi P, Asensio M, Herrero M, Núñez K, Cañibano E, Merino JC. Control of branches distribution in linear PE copolymers using fibrillar nanoclay as support of catalyst system. Polymer (Guildf). 2020;202(June).
17. Herrero M, Núñez K, Gallego R, Merino JC, Pastor JM. Control of molecular weight and polydispersity in polyethylene/needle-like shaped clay nanocomposites obtained by in situ polymerization with metallocene catalysts. Eur Polym J [Internet]. 2016;75:125–41. Available from: http://dx.doi.org/10.1016/j.eurpolymj.2015.12.005
18. Kaminsky W. Polyole fi n-nanocomposites with special properties by in-situ polymerization. Chem Sci Eng. 2018;12(3):555–63.
19. Cromer BM, Scheel S, Luinstra GA, Coughlin EB, Lesser AJ. In-situ polymerization of isotactic polypropylene-nanographite nanocomposites. Polymer (Guildf) [Internet]. 2015;80:275–81. Available from: http://dx.doi.org/10.1016/j.polymer.2015.09.074
20. Palaznik OM, Nedorezova PM, Shevchenko VG, Krasheninnikov VG, Monakhova T V., Arbuzov AA. Synthesis and Properties of Polymerization-Filled Composites Based on Polypropylene and Single-Wall Carbon Nanotubes. Polym Sci - Ser B. 2021;63(2):138–51.
21. Cardoso RS, Aguiar VO, Marques M de F V. Masterbatches of polypropylene/clay obtained by in situ polymerization and melt-blended with commercial polypropylene. J Compos Mater. 2017;51(25):3547–56.
22. Asensio M, Herrero M, Núñez K, Gallego R, Merino JC, Pastor JM. In situ polymerization of isotactic polypropylene sepiolite nanocomposites and its copolymers by metallocene catalysis. Eur Polym J [Internet]. 2018;100:278–89. Available from: https://doi.org/10.1016/j.eurpolymj.2018.01.034
23. Polshchikov S V., Nedorezova PM, Komkova OM, Klyamkina AN, Shchegolikhin AN, Krasheninnikov VG, et al. Synthesis by polymerization in situ and properties of composite materials based on syndiotactic polypropylene and carbon nanofillers. Nanotechnologies Russ. 2014;9(3–4):175–83.
24. Núñez K, Gallego R, Pastor JM, Merino JC. Applied Clay Science The structure of sepiolite as support of metallocene co-catalyst during in situ polymerization of polyole fi n ( nano ) composites. Appl Clay Sci [Internet]. 2014;101:73–81. Available from: http://dx.doi.org/10.1016/j.clay.2014.07.020
25. C. Vasile. Handbook of polyolefins, second edition. New York: Mercel Dekker. 2000.
26. Núñez K, Tanasi P, Asensio M, Herrero M, Alonso LE, Guerrero J, et al. In situ polymerisation of stereospecific propylene nanocomposites blends. Optimising mechanical properties. Polymer (Guildf). 2022;240(August 2021).
27. TOBIAS S. HALBACH, YI THOMANN RM. Boehmite Nanorod-Reinforced-Polyethylenes and Ethylene/1-Octene Thermoplastic Elastomer Nanocomposites Prepared by In Situ Olefin Polymerization and Melt Compounding. J Polym Sci Part A Polym Chem. 2008;46:2755–65.
28. Chen Z, Brookhart M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts. Acc Chem Res. 2018;51(8):1831–9.
29. Keyes A, Basbug Alhan HE, Ordonez E, Ha U, Beezer DB, Dau H, et al. Olefins and Vinyl Polar Monomers: Bridging the Gap for Next Generation Materials. Angew Chemie - Int Ed. 2019;58(36):12370–91.
30. Bortolussi F, Broyer JP, Spitz R, Boisson C. Synthesis of silica-supported metallocene catalysts for olefin polymerization. Macromol Chem Phys. 2002;203(17):2501–7.
31. Mingkwan Wannaborworn PP and BJ. Observation of Different Catalytic Activity of Various 1-Olefins during Ethylene/1-Olefin Copolymerization with Homogeneous Metallocene Catalysts. Molecules. 2011;16:373–83.
Document information
Published on 10/01/23
Accepted on 27/06/22
Submitted on 27/06/22
Volume 07 - COMUNICACIONES MATCOMP21 (2022), Issue Núm. 3 - Materiales y Estructuras - Modelos Numéricos, 2023
DOI: 10.23967/r.matcomp.2023.01.02
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?