Abstract
Background
Ceramide-1-phosphate (C1P) is a phosphorylated form of ceramide. While ceramide is known to be an inducer of apoptosis of cochlear hair cells in cisplatin ototoxicity, little is known about the function of C1P in cochlear diseases.
Purpose
The present study was designed to examine whether C1P could protect cochlear hair cells against cisplatin ototoxicity.
Materials and methods
Explants of cochlear basal turns collected from C57BL/6J mice at postnatal days 3–5 were used in all experiments. Cochlear explants were exposed to 5 or 10 μM cisplatin for 48 h to assess the effects of C1P, NVP-231 (a ceramide kinase inhibitor), or ceramide. Western blotting of pAkt/Akt and pMAPK/MAPK was examined to check whether this pathway was modulated by C1P.
Results
C1P activated the Akt and MAPK pathway and significantly reduced cochlear cell death induced by cisplatin. Coadministration of cisplatin and ceramide significantly increased cochlear hair cell death. In addition, when treating cochlear hair cells with NVP-231 in the presence of cisplatin or ceramide, a remarkable increase in apoptosis of hair cells was observed.
Conclusion
The present findings confirmed the protective effects of C1P in the cisplatin ototoxicity. The balance between ceramide and C1P may play a critical role in the determination of hair cell fate in cisplatin ototoxicity.
Keywords
Ceramide ; Ceramide-1-phosphate ; Ceramide kinase ; Cisplatin
1. Introduction
Cochlear hair cells have a crucial function to convert sound signals to electric signals in the auditory system; however, they are very sensitive to harmful stimulations such as cisplatin used in chemotherapy.
Ceramide is generally formed either via de novo synthesis from serine and palmitoyl-CoA or via hydrolysis of sphingomyeline by sphingomyelinase [1] and [17] . It was demonstrated that cisplatin activates sphingomyelinase, which triggers the release of ceramide [18] . Although ceramide is known to be an inducer of apoptosis in aminoglycoside ototoxicity [19] , the effects of ceramides on cisplatin ototoxicity have never been examined.
Ceramide-1-phosphate (C1P) is synthesized by phosphorylation of ceramide in mammalian cells [6] . Currently, ceramide kinase (CERK) is the only known mammalian enzyme to have this function. Since the first biological activity of C1P relating to DNA synthesis and cell division was identified [10] and [11] , researchers have found that this phospholipid was attributed to various vital functions, such as macrophage proliferation and migration [7] and [14] , phagocytosis [15] , inflammation [22] and [23] and cell protection [12] and [13] . However, it has not been revealed yet whether C1P could inhibit cisplatin-induced cochlear hair cell death.
Induction of cell death is a complex process and tightly regulated. In the present study we hypothesized that C1P could be an antiapoptotic molecule and that targeted ceramide/C1P balance could interfere cochlear cell survival.
2. Methods
2.1. Culture technique
2.1.1. Cochlear explants
The lower basal turn of the organ of Corti was dissected from C57BL/6J mouse on postnatal days 3 (P3) to 5 (P5) and cultured according to the methods of Van de Water and Ruben [29] and Sobkowicz et al. [25] . All animal procedures were carried out according to the guidelines of the Laboratory Animal Research Center of Tsukuba University.
2.1.2. Cisplatin treatment
Cochlear explants were maintained in a culture medium containing Dul-becco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum (FBS), 25 mM HEPES, and 30 U/mL penicillin. They were cultured in an incubator at 37 °C with 5% CO2 at 95% humidity. Cochlear explants were maintained in the culture medium overnight (8–12 h) and then were exposed to the culture medium containing cisplatin (Nichi-Iko, Sogawa, Japan) for 48 h [27] .
Cisplatin-induced ototoxicity was characterized by the missing of cochlear outer hair cells at the basal turn of the organ of Corti. Before conducting this study, we examined the damage of cochlear outer hair cells by exposing cochlear explants to several concentrations of cisplatin from 1 to 50 μM. The concentrations of cisplatin at 5 and 10 μM were chosen for the present experiments.
2.1.3. C1P treatment
C1P (Sigma, St. Louis, MO, USA) was initially dissolved in ethanol at a concentration of 2 mg/ml, and then diluted in the culture medium to the final concentrations before use. After the cochlear explants were stabilized in the culture medium overnight, each group (from 9 to 18 cochlear explants) was exposed to culture media containing 10 μM cisplatin plus various concentrations of C1P (from 1 to 100 μM) for 48 h.
2.1.4. Ceramide treatment
C16-ceramide (LKT Laboratories, St. Paul, MN, USA) was dissolved in ethanol at a concentration of 5 mg/ml. The effect of ceramide alone on cochlear explants was tested using concentrations from 10 to 500 μM. Later, the combination of 5 μM cisplatin and various concentrations of ceramide (from 10 to 500 μM) was examined. In another condition, 500 μM ceramide was selected to mix with various concentrations of NVP-231 (from 10 to 200 μM), and this combination was applied to assess the survival of cochlear outer hair cells. In all experiments, cochlear explants were stabilized in the normal culture medium overnight before treated in different conditions for 48 h.
2.1.5. NVP-231 treatment
NVP-231 was dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO, USA) at a concentration of 10 mg/ml. After being stabilized in the culture medium overnight, cochlear explants were exposed to culture media containing 5 μM cisplatin plus various concentrations of NVP-231 (from 0.1 to 10 μM) for 48 h. Using the similar experimental model as above, cochlear outer hair cells were treated in culture media with 500 μM ceramide plus NVP-231 ranging from 10 to 200 μM.
2.2. Cytochemistry
At the end of the tissue culture, cochlear explants were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min and then permeabilized with 5% Triton X-100 (Sigma, St. Louis, MO,USA) in PBS with 10% fetal bovine serum for 10 min. The specimens were stained with phalloidin using a conjugated Alexa Fluor probe (1:100, Molecular Probes, Carlsbad, CA, USA) at room temperature for 1 h [27] . Phalloidin is a specific marker for cellular F-actin and labels stereociliary arrays and the cuticular plates of hair cells. The specimens were observed using a confocal microscope (FluoView F10i, Olympus, Center Valley, PA, USA). Hair cells were characterized as missing if no stereocilia or no cuticular plates were observed by phalloidin staining. Residue of cochlear outer hair cells was expressed as a percentage and the results of each the groups were compared [27] .
2.3. Western blots
The organs of Corti were treated in culture media alone or in culture media containing either 10 μM cisplatin or 10 μM cisplatin plus 100 μM C1P for 24 or 48 h. Explants were then homogenized in Pro-Prep protein extraction solution (iNtRON, Kyungki, Korea) following the product’s protocol. After being centrifuged at 13.000 rpm at 4 °C for 10 min, the supernatant was transferred to a fresh tube and the protein content was measured by using protein assay rapid kit (Wako, Osaka, Japan). Equal amounts of lysate protein (20 μg) were electrophoretically separated on 4–20% mini-PROTEAN TGX precast polyacrylamide gel (Bio-Rad, Hercules, CA, USA) and transferred to Hybond polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, UK). After blocking, each membrane was primarily treated with polyclonal antibodies Akt (1:500), pAkt (1:500), MAPK (1:500), or pMAPK (1:500) overnight at 4 °C. Horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2000) was secondarily treated for 1 h at room temperature. The antibodies described above were purchased from Cell Signaling Technology (Beverly, MA, USA). Chemiluminescent detection was enhanced with ECL kit (GE Healthcare, Buckinghamshire, UK) and the immunoblot images were obtained with the ImageQuant LAS4000 mini imager (GE Healthcare, Buckinghamshire, UK). Densitometric analysis was done from at least 3 independent experiments by using ImageJ software (Nation Institutes of Health).
2.4. Data analysis
All data were expressed as the means ± SDs. Statistical analysis was performed by t -test or one-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests, as required (SPSS 20). Probability values less than 0.05 were considered significant.
3. Results
3.1. Control study
The effects of 100 μM C1P, 10 μM NVP-231 or solvents alone (1% ethanol and 0.04% DMSO) on cochlear hair cells were examined. After 48-h exposure to above agents, all of cochlear outer hair cells remained intact. In addition, the interactions between cisplatin and solvents were also checked. There were no significant differences in cochlear hair cell loss between explants treated with cisplatin alone and one treated with cisplatin plus either 1% ethanol or 0.04% DMSO; the tested concentrations of cisplatin were 5 and 10 μM (data not shown).
3.2. C1P and the survival of outer hair cell in cisplatin ototoxicity
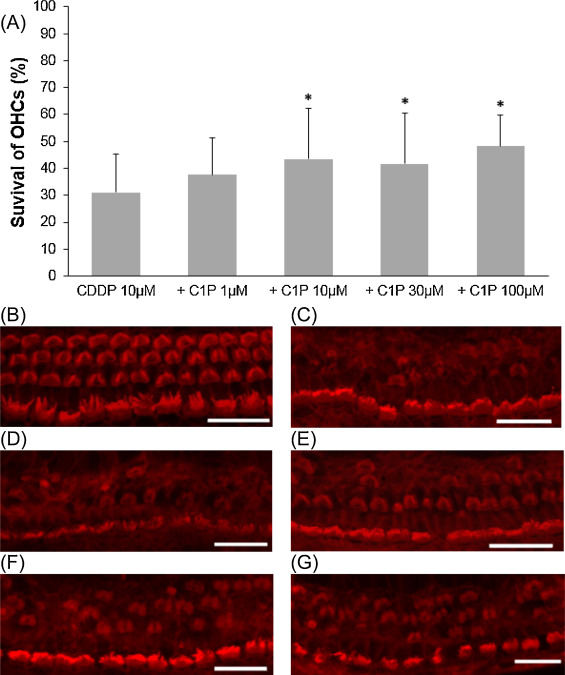
We examined the effect of C1P on cisplatin-induced outer hair cell damage. The results revealed a dose-dependent relationship between C1P treatment and the survival of cochlear outer hair cells. When compared with cisplatin treatment alone, the addition of C1P treatment significantly increased the survival of cochlear outer hair cells at 10 μM C1P or higher (Fig. 1 ; ANOVA followed by Bonferroni post hoc test; 10 μM-C1P group, n = 16, P = 0.014; 30 μM-C1P, n = 18, P = 0.03; 100 μM-C1P, n = 9, P = 0.007; n is the number of cochlear explants).
|
|
|
Fig. 1. Effects of C1P on the survival of cochlear outer hair cell in cisplatin ototoxicity. (A) Quantitative analysis of cochlear outer hair cells. The survival of cochlear outer hair cells significantly increased at 10 μM C1P or higher (ANOVA followed by Bonferroni post hoc test, *P < 0.05). (B − G) Representative photographs of each group (scale bar 20 μm). (B) 100 μM C1P alone. (C) 10 μM cisplatin alone. (D) 10 μM cisplatin and 1 μM C1P. (E) 10 μM cisplatin and 10 μM C1P. (F) 10 μM cisplatin and 30 μM C1P. (G) 10 μM cisplatin and 100 μM C1P. CDDP: cisplatin. |
3.3. C1P and phosphorylation of akt and MAPK
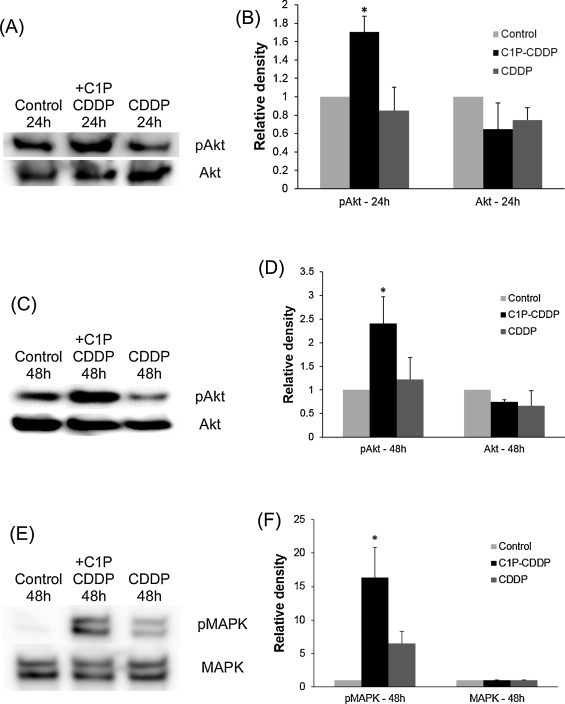
The above results suggested that C1P could inhibit cochlear outer hair cell death induced by cisplatin. Next, Western blots were conducted to determine whether this effect of C1P involves the stimulation of the PI3-K/Akt or ERK/MAPK pathway. Cochlear explants were treated in 3 different conditions, including the culture medium alone, 10 μM cisplatin, or 10 μM cisplatin plus 100 μM C1P. Immunoblot images revealed strong activations of Akt (Fig. 2 A and C) and MAPK (Fig. 2 E) in cochlear explants treated with C1P. In addition, densitometric analysis also revealed significant increases of pAkt in a group of C1P addition compared to a group without C1P for 24 h (Fig. 2 B; t -Test, P = 0.005) or 48 h (Fig. 2 D; t -Test, P = 0.025). The signal of pMAPK was also significantly increased when C1P was added for 48 h (Fig. 2 F; t -Test, P = 0.015)
|
|
|
Fig. 2. Representative Western blots of pAkt, total Akt, pMAPK and total MAPK. Comparisons were conducted among 3 groups: control group (no cisplatin), 10 μM cisplatin plus 100 μM C1P group, and 10 μM cisplatin group. (A) Chemiluminescent detection of pAkt and Akt with 24-h treating time. (C) Chemiluminescent detection of pAkt and Akt with 48-h treating time. (E) Chemiluminescent detection of pMAPK and MAPK with 48-h treating time. (B, D, F) C1P significantly increased densitometric signals of pAkt and pMAPK in comparison to groups without C1P (t-Test, P < 0.05). Densitometric values were normalized to values of control group. pAkt and pMAPK have been referenced to total Akt and total MAPK, respectively. Data were obtained from three independent experiments. CDDP: cisplatin. |
3.4. The effect of ceramide on cisplatin-induced cochlear hair cell death
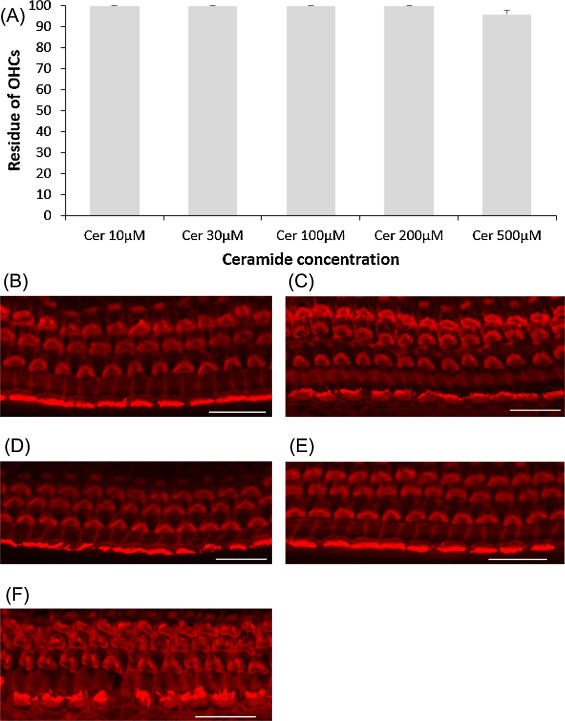
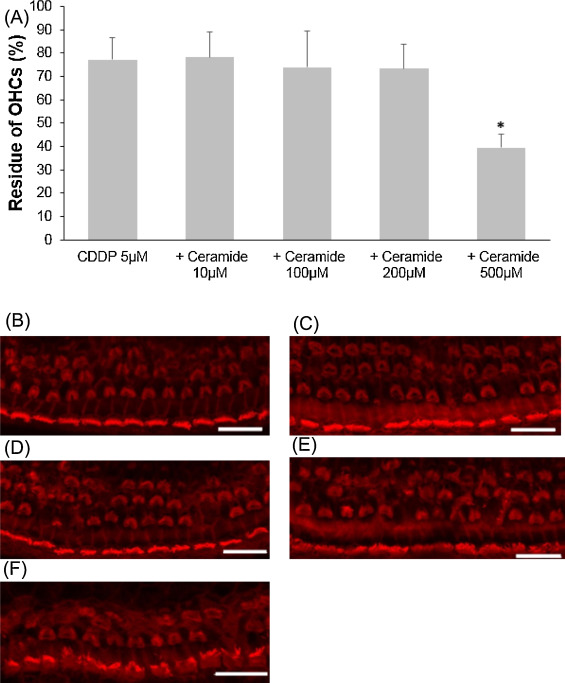
Ceramide is a regulator of cellular apoptosis, and this experiment was conducted to show whether or not ceramide amplifies cisplatin-induced hair cell death. First, ceramide alone was examined on cochlear explants. All cochlear outer hair cells were intact at 200 μM ceramide or lower, and very few hair cells were lost at 500 μM (Fig. 3 .). Later, several concentrations of ceramide as above were tested with 5 μM cisplatin addition. Only the condition of 500 μM ceramide plus 5 μM cisplatin significantly decreased the number of cochlear outer hair cells (Fig. 4 ; ANOVA followed by Bonferroni post hoc test; n = 9, P < 0.001 at 500 μM).
|
|
|
Fig. 3. Effects of ceramide on cochlear hair cell. (A) Quantitative analysis of cochlear outer hair cells. All cochlear outer hair cells were intact with ceramide concentration lower than 200 μM and few were lost at 500 μM. (B − F) Representative photographs of each group (scale bar 20 μm). (B) 10 μM ceramide. (C) 30 μM ceramide. (D) 100 μM ceramide. (E) 200 μM ceramide. (F) 500 μM ceramide. Cer: ceramide. |
|
|
|
Fig. 4. Effects of ceramide on cisplatin-induced cochlear hair cell death. (A) Quantitative analysis of cochlear outer hair cells. The residue of cochlear outer hair cells decreased significantly at 500 μM ceramide (ANOVA followed by Bonferroni post hoc test, *P < 0.001). (B − F) Representative photographs of each group (scale bar 20 μm). (B) 5 μM cisplatin alone. (C) 5 μM cisplatin and 10 μM ceramide. (D) 5 μM cisplatin and 100 μM ceramide. (E) 5 μM cisplatin and 200 μM ceramide. (F) 5 μM cisplatin and 500 μM ceramide. CDDP: cisplatin. |
3.5. CERK inhibitor and cochlear hair cell death induced by cisplatin or ceramide
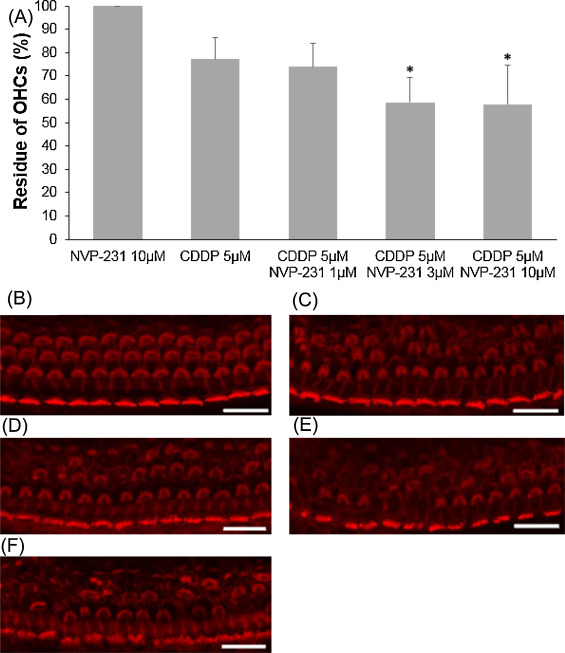
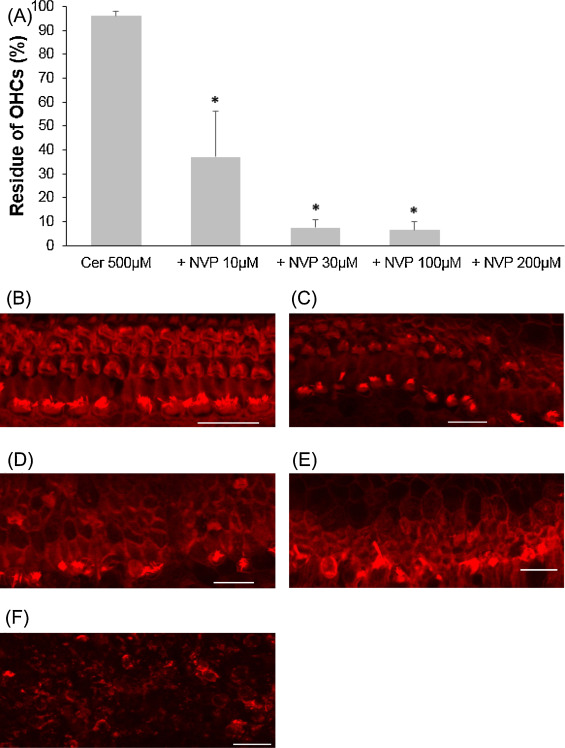
CERK converts ceramide to C1P and contributes to the balance of the C1P and ceramide levels. NVP-231, a selective CERK inhibitor, was used to evaluate the involvement of CERK when cochlear outer hair cells exposure to cisplatin or ceramide. At first, 5 μM cisplatin was mixed with to several concentrations of NVP-231. Outer hair cell death induced by 5 μM cisplatin was significantly exacerbated in the presence of NVP-231 at 3 μM or higher (Fig. 5 ; ANOVA followed by Bonferroni post hoc test; 3 μM NVP-231 group, n = 10, P = 0.008; 10 μM NVP-231 group, n = 10, P = 0.005). Later, in a different condition, 500 μM ceramide was combined with several concentrations of NVP-231. Outer hair cell death was severely damaged, starting with 10 μM NVP-231, and hair cells were completely unable to identify at 200 μM (Fig. 6 ; ANOVA followed by Bonferroni post hoc test; 10 μM NVP-231 group, n = 9, P = 0.000; 30 μM NVP-231 group, n = 10, P = 0.000; 100 μM NVP-231 group, n = 9, P = 0.000)
|
|
|
Fig. 5. Effects of NVP-231 on cisplatin-induced cochlear hair cell death. (A) Quantitative analysis of cochlear outer hair cells. The survival of cochlear outer hair cells was statistically decreased through the effect of 3 μM NVP-231 or higher (ANOVA followed by Bonferroni post hoc test, *P < 0.05). (B − F) Representative photographs of each group (scale bar 20 μm). (B) 10 μM NVP-231 alone. (C) 5 μM cisplatin alone. (D) 5 μM cisplatin and 1 μM NVP-231. (E) 5 μM cisplatin and 3 μM NVP-231. (F) 5 μM cisplatin and 10 μM NVP-231. CDDP: cisplatin. |
|
|
|
Fig. 6. Effects of NVP-231 on cochlear hair cell treated with ceramide. (A) Quantitative analysis of cochlear outer hair cells. The survival of cochlear outer hair cells was statistically decreased when NVP-231, starting from 10 to 200 μM, was added to culture media containing 500 μM ceramide (ANOVA followed by Bonferroni post hoc test, *P = 0.000). (B − F) Representative photographs of each group (scale bar 20 μm). (B) 500 μM ceramide alone. (C) 500 μM ceramide and 10 μM NVP-231. (D) 500 μM ceramide and 30 μM NVP-231. (E) 500 μM ceramide and 100 μM NVP-231. (F) 500 μM ceramide and 200 μM NVP-231; all explants were severely damaged. Cer: ceramide. |
4. Discussion
It has been demonstrated that C1P participates in several vital pathophysiological functions including regulation of cell growth and survival, stimulation of DNA synthesis and cell division, and inhibition of cellular apoptosis [3] , [9] and [11] . In the present study, its involvement in cisplatin-induced hair cell death was investigated. By using C1P, cisplatin-induced cochlear outer hair cell death was successfully suppressed in our experiments.
In the first few hours of cisplatin exposure, cochlear hair cells enhance their defence mechanisms by inducing the upregulation of several anti-apoptotic genes that are associated with the NF-κB pathway, caspase recruitment domain family, IAP family, Bcl-2 family, and p53 signaling [5] . These signaling pathways are also known to be stimulated by the PI3-K/Akt pathway. Our study revealed that activation of the PI3-K/Akt pathway was at least one of the mechanisms in which C1P promotes cochlear hair cell survival. Besides, the activity of sphingomyelinase, which converts sphingomyeline to ceramide, was reportedly restrained by the activation of this pathway [28] and as a result prevented the increase of ceramide [20] . Additionally, the activation of MAPK/ERK pathway, which is related to cell growth, could be another explanation of cochlear hair cell survival in our study. In fact, this activation was reported in experimental models of C1P with osteoblastic cells [3] and macrophages [8] .
Ceramide causes the death of neuronal cells in the central and peripheral nervous systems [2] , [4] and [30] . Although its precise mechanism remains unclear, it is currently thought that ceramide induces cellular apoptosis via activation of multiple pathways (e.g., PKC, PP2A, etc.) [24] . Ceramide reportedly inhibits the PI3-K/Akt pathway [31] . In the present study, ceramide alone had least effects on cochlear hair cells. Co-administration of ceramide and cisplatin only accelerated the death of cochlear outer hair cells at very high concentration. In mammalian cells, CERK directly phosphorylates ceramide to synthesize C1P and is currently the only known enzyme to have this function [26] . Therefore, we hypothesized that cochlear hair cells might have a high intrinsic activity of CERK, which rapidly phosphorylates ceramide resulting in increase of C1P synthesis; thus, cochlear hair cells were protected from the damage of high ceramide concentration. When treating cochlear hair cells with NVP-231 (a CERK inhibitor) in the presence of cisplatin or ceramide, we observed remarkable increases of apoptosis. These results confirmed our hypothesis above and explained partly the central role of C1P in governing cochlear hair cell fate.
Taken together our results suggest the importance of the balance between ceramide and C1P. On one hand, ceramides triggers apoptosis via several pathways including blocking the PI3-K/Akt; on the other hand, C1P inhibits this process by activating the PI3-K/Akt pathway and increase phosphorylation of MAPK. In short, our present study suggests that at least the balance of ceramide and C1P regulates cochlear hair cell fate, and any circumstances that alter this balance (e.g., a CERK inhibitor) will affect the survival of cochlear hair cell.
Since the discovery of CERK over 10 years ago, some evidences showed that inhibition of CERK might enhance the efficacy of anti-cancer therapies. When the activity of CERK is abolished, it greatly increases the susceptibility of tumor cell lines to low-dose UV irradiation [16] . Furthermore, it is suggested that the inhibition of CERK will overcome the issue of chemotherapeutic resistance by rising ceramide levels in combination of chemotherapies [21] . However, the inhibition of CERK needs to be approached with great caution, because of a possibility of an unbalance between ceramide and C1P. The elevation of ceramide level would possibly render other normal cells more vulnerable to the treatment − particularly cochlear hair cells in cisplatin ototoxicity, as the findings of our study.
In summary, this present study provides evidences that C1P regulates cochlear hair cell survival in cisplatin ototoxicity through the PI3-K/Akt and MAPK pathway.
Conflict of interest
The authors declare that they have no conflict of interest
Acknowledgement
This work was supported by a Grant-in-aid for Scientific Research (C) 24592541 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- [1] D.A. Brown, E. London; Functions of lipid rafts in biological membranes; Annu. Rev. Cell Dev. Biol., 14 (1998), pp. 111–136
- [2] A. Carpinteiro, C. Dumitru, M. Schenck, E. Gulbins; Ceramide-induced cell death in malignant cells; Cancer Lett., 264 (2008), pp. 1–10
- [3] L.C. Carpio, E. Stephan, A. Kamer, R. Dziak; Sphingolipids stimulate cell growth via MAP kinase activation in osteoblastic cells; Prostaglandins Leukot. Essent. Fatty Acids, 61 (1999), pp. 267–273
- [4] L. Cavallini, R. Venerando, G. Miotto, A. Alexandre; Ganglioside GM1 protection from apoptosis of rat heart fibroblasts; Arch. Biochem. Biophys., 370 (1999), pp. 156–162
- [5] D.l. Ding, W. Ping, J. Haiyan, D. Coling, R. Salvi; Gene expression in cisplatin ototoxicity and protection with p53 inhibitor; J. Otol., 4 (2009), pp. 61–70
- [6] K.A. Dressler, R.N. Kolesnick; Ceramide 1-phosphate, a novel phospholipid in human leukemia (HL-60) cells. Synthesis via ceramide from sphingomyelin; J. Biol. Chem., 265 (1990), pp. 14917–14921
- [7] P. Gangoiti, M.H. Granado, L. Arana, A. Ouro, A. Gomez-Munoz; Activation of protein kinase C-alpha is essential for stimulation of cell proliferation by ceramide 1-phosphate; FEBS Lett., 584 (2010), pp. 517–524
- [8] P. Gangoiti, M.H. Granado, S.W. Wang, J.Y. Kong, U.P. Steinbrecher, A. Gomez-Munoz; Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways; Cell. Signal., 20 (2008), pp. 726–736
- [9] A. Gomez-Munoz; Ceramide-1-phosphate: a novel regulator of cell activation; FEBS Lett., 562 (2004), pp. 5–10
- [10] A. Gomez-Munoz, P.A. Duffy, A. Martin, L. O’Brien, H.S. Byun, R. Bittman, D.N. Brindley; Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides; Mol. Pharmacol., 47 (1995), pp. 833–839
- [11] A. Gomez-Munoz, L.M. Frago, L. Alvarez, I. Varela-Nieto; Stimulation of DNA synthesis by natural ceramide 1-phosphate; Biochem. J., 325 (Pt. 2) (1997), pp. 435–440
- [12] A. Gomez-Munoz, J.Y. Kong, K. Parhar, S.W. Wang, P. Gangoiti, M. Gonzalez, S. Eivemark, B. Salh, V. Duronio, U.P. Steinbrecher; Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway; FEBS Lett., 579 (2005), pp. 3744–3750
- [13] A. Gomez-Munoz, J.Y. Kong, B. Salh, U.P. Steinbrecher; Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages; J. Lipid Res., 45 (2004), pp. 99–105
- [14] M.H. Granado, P. Gangoiti, A. Ouro, L. Arana, M. Gonzalez, M. Trueba, A. Gomez-Munoz; Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor; Cell. Signal., 21 (2009), pp. 405–412
- [15] V.T. Hinkovska-Galcheva, L.A. Boxer, P.J. Mansfield, D. Harsh, A. Blackwood, J.A. Shayman; The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion; J. Biol. Chem., 273 (1998), pp. 33203–33209
- [16] S.Y. Hsieh, C.Y. Hsu, J.R. He, C.L. Liu, S.J. Lo, Y.C. Chen, H.Y. Huang; Identifying apoptosis-evasion proteins/pathways in human hepatoma cells via induction of cellular hormesis by UV irradiation; J. Proteome Res., 8 (2009), pp. 3977–3986
- [17] R.N. Kolesnick, F.M. Goni, A. Alonso; Compartmentalization of ceramide signaling: physical foundations and biological effects; J. Cell. Physiol., 184 (2000), pp. 285–300
- [18] S. Lacour, A. Hammann, S. Grazide, D. Lagadic-Gossmann, A. Athias, O. Sergent, G. Laurent, P. Gambert, E. Solary, M.T. Dimanche-Boitrel; Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells; Cancer Res., 64 (2004), pp. 3593–3598
- [19] B. Nishimura, K. Tabuchi, M. Nakamagoe, A. Hara; The influences of sphingolipid metabolites on gentamicin-induced hair cell loss of the rat cochlea; Neurosci. Lett., 485 (2010), pp. 1–5
- [20] S. Noda, S. Yoshimura, M. Sawada, T. Naganawa, T. Iwama, S. Nakashima, N. Sakai; Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells; J. Neurooncol., 52 (2001), pp. 11–21
- [21] B. Ogretmen, Y.A. Hannun; Biologically active sphingolipids in cancer pathogenesis and treatment; Nat. Rev. Cancer, 4 (2004), pp. 604–616
- [22] B.J. Pettus, A. Bielawska, S. Spiegel, P. Roddy, Y.A. Hannun, C.E. Chalfant; Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release; J. Biol. Chem., 278 (2003), pp. 38206–38213
- [23] B.J. Pettus, A. Bielawska, P. Subramanian, D.S. Wijesinghe, M. Maceyka, C.C. Leslie, J.H. Evans, J. Freiberg, P. Roddy, Y.A. Hannun, C.E. Chalfant; Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2; J. Biol. Chem., 279 (2004), pp. 11320–11326
- [24] P.P. Ruvolo; Intracellular signal transduction pathways activated by ceramide and its metabolites; Pharmacol. Res., 47 (2003), pp. 383–392
- [25] H.M. Sobkowicz, J.M. Loftus, S.M. Slapnick; Tissue culture of the organ of Corti; Acta Otolaryngol. Suppl., 502 (1993), pp. 3–36
- [26] M. Sugiura, K. Kono, H. Liu, T. Shimizugawa, H. Minekura, S. Spiegel, T. Kohama; Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization; J. Biol. Chem., 277 (2002), pp. 23294–23300
- [27] K. Tabuchi, K. Pak, E. Chavez, A.F. Ryan; Role of inhibitor of apoptosis protein in gentamicin-induced cochlear hair cell damage; Neuroscience, 149 (2007), pp. 213–222
- [28] F.D. Testai, M.A. Landek, R. Goswami, M. Ahmed, G. Dawson; Acid sphingomyelinase and inhibition by phosphate ion: role of inhibition by phosphatidyl-myo-inositol 3,4,5-triphosphate in oligodendrocyte cell signaling; J. Neurochem., 89 (2004), pp. 636–644
- [29] T. Van de Water, R.J. Ruben; Growth of the inner ear in organ culture; Ann. Otol. Rhinol. Laryngol., 83 (1974), pp. 1–16
- [30] C.L. Yen, M.H. Mar, S.H. Zeisel; Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin accumulation of ceramide and diacylglycerol, and activation of a caspase; FASEB J., 13 (1999), pp. 135–142
- [31] W. Zundel, L.M. Swiersz, A. Giaccia; Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide; Mol. Cell. Biol., 20 (2000), pp. 1507–1514
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?