Summary
Issues related to small-for-size grafts in living donor liver transplantation (LDLT) are highly important. The neutrophil lymphocyte ratio (NLR) has been reported to be an inexpensive index of systemic inflammation for various diseases. We retrospectively evaluated the relationship between NLR and clinical course of 61 adult LDLT recipients in our institute until post-operative day 14. Patients were classified into two groups based on the graft volume divided by standard liver volume, as over 35% of graft volume divided by standard liver volume (GV/SLV) (Group L; n = 55) and under 35% of GV/SLV (Group S; n = 6). No differences were seen in background of the patients between the two groups. Also, absolute neutrophil, lymphocyte and platelet counts in both the groups showed no significant differences. In contrast, the NLR between the groups differed significantly from post-operative day 3 to 10, being higher in the Group S. In addition, the incidence of prolonged hyperbilirubinemia and small for size graft syndrome differed significantly between the two groups. Therefore, the elevation of post-operative NLR in the smaller graft group reflect suggestive pathophysiology of endothelial injuries that related to small for size graft syndrome in LDLT.
Keywords
complete blood count;endothelial injury;small-for-size graft
1. Introduction
Many reports have described issues associated with graft size in living-donor liver transplantation (LDLT).1 ; 2 Graft size affects the small-for-size (SFS) graft syndrome, which is often catastrophic and needs to be avoided.3 The principal pathogenesis of the SFS syndrome is thought to be excessively increased portal flow and the subsequent induction of graft sinusoidal endothelial injury.2 However, the symptoms of the SFS syndrome cannot be completely avoided, even when an appropriate ratio of graft size to portal inflow is obtained. Therefore, a greater understanding of the underlying pathophysiology is important for overcoming the SFS syndrome.
Complete blood count is an inexpensive and indispensable test following major surgeries, including LDLT. Thus far, the platelet count and its time-serial changes have been the focus of attention, given its reported relationship with postoperative morbidity and mortality.4 Similarly, the neutrophil-to-lymphocyte ratio (NLR) is an inexpensive index of systemic inflammation.5 Preoperative NLR has been investigated as a prognostic factor of hepatocellular carcinoma (HCC) in LDLT recipients.6 In addition, a relationship between prognosis and NLR has been reported in patients with colorectal, lung, and ovarian cancers, as well as in HCC patients.7; 8; 9 ; 10 Further, NLR can predict the survival in patients of acute coronary syndrome treated by percutaneous coronary intervention and coronary artery bypass grafting.11 ; 12 However, to date, no reports have analyzed the postoperative NLR in LDLT recipients. Here, we describe a retrospective pilot study to evaluate the relationship between NLR and adult SFS grafts, along with an analysis of other clinical factors.
2. Methods
Between January 1999 and December 2013, 61 patients underwent their first adult LDLT at Kanazawa University Hospital, Kanazawa, Japan. These patients were included in the present study after obtaining an approval from the Institutional Review Board of Kanazawa University Hospital. All living donors were evaluated by contrast-enhanced abdominal computed tomography with using three-dimensional image-analyzing system (SYNAPSE VINCENT; Fuji Film, Tokyo, Japan). The results of the computed tomography were used to calculate whole-liver volumetry, liver graft volume, and residual liver in the donor. The standard liver volume was calculated using the formula developed by Urata et al.13 The actual graft weight of the procured graft was measured on the back table in the operating room and was defined as graft volume (GV). Then, the graft size was evaluated as the GV/standard liver volume (GV/SLV) ratio and the graft-to-recipient body-weight ratio.
The transplant procedures for both donors and recipients have previously been reported.14 Hepatic arterial reconstruction was performed using a surgical microscopic procedure. Biliary reconstruction was routinely conducted in a duct-to-duct fashion. Portal vein pressure during surgery was not measured, and concomitant splenectomy for inflow modulation was not performed during this period.
After the transplant, the immunosuppressive therapy started with tacrolimus (Prograf; Astellas Pharma, Tokyo, Japan) and corticosteroids. The tacrolimus dose was adjusted to achieve a trough level of 10–15 ng/mL for 2 weeks following the transplant. Thereafter, the target trough level was gradually reduced to approximately 7 ng/mL. The corticosteroids were administered as an initial dose of 2 mg/kg/d, which was tapered gradually. The principle of postoperative managements about the transplant recipient was not differed according to the graft size.
The recorded clinical data, including preoperative general demographics, the model for end-stage liver disease (MELD) score, graft weight, postoperative changes in complete blood count, prothrombin time–international normalized ratio, total bilirubin levels, C-reactive protein, and total amount of drained ascites fluid at postoperative Days (POD) 1, POD 3, POD 5, POD 7, POD 10, and POD 14 were analyzed. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count. The MELD score was calculated with the formula reported by Kamath et al.15 Further, the occurrence of adverse clinical events and complications, including infections, acute cellular rejection, or relaparotomy, was retrospectively analyzed during the postoperative hospital stay for LDLT. Both pre- and postoperative infectious complications included surgical site infection defined as above Grade IIIa of the Clavien–Dindo classification, clinically treated pneumonia, bacteremia, and other infectious episodes.16 Acute cellular rejection included clinically suspected and steroid-treated cases, as well as histologically proven ones. The SFS syndrome is defined as the criteria of the report from Soejima et al.3
To analyze the relationship between SFS graft and other clinical factors, the patients were divided into two groups according to the GV/SLV, as over 35% GV/SLV (Group L) and under 35% GV/SLV (Group S). The parametric variables were compared using unpaired Student t test, while the nonparametric variables were compared using Chi-square analysis. The survival probability of the recipients was determined by the Kaplan–Meier method. A p value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 20 (SPSS Inc., Chicago, IL, USA).
3. Results
The baseline characteristics of the recipients, donors, grafts, and operations are listed in Table 1. No differences were seen with respect to age, gender, or MELD scores between the two groups. The indications of liver transplantation differed significantly between the two groups. Further, the mean GV/SLV and graft-to-recipient body-weight ratio between the two groups showed significant differences, being 48.8 ± 9.9% and 0.98 ± 0.26% in Group L, and 32.5 ± 2.0% (p < 0.001) and 0.62 ± 0.06% (p < 0.01) in Group S, respectively.
| Factors | Group L (n = 55) | Group S (n = 6) | p |
|---|---|---|---|
| Recipient | |||
| Age (y) | 52.9 ± 9.7 | 50.7 ± 8.7 | 0.58 |
| Male/female | 33/22 | 3/3 | 0.68 |
| MELD score | 19.1 ± 12.3 | 20.2 ± 3.7 | 0.83 |
| Indication | 0.03 | ||
| Cholestatic diseases | 13 | 0 | |
| Fulminant hepatic failure | 1 | 1 | |
| HCC | 27 | 0 | |
| Liver cirrhosis | 12 | 5 | |
| Others | 2 | 0 | |
| Donor and graft | |||
| Age (y) | 46.5 ± 6.9 | 40.2 ± 13.0 | 0.25 |
| Male/female | 32/23 | 4/2 | 0.70 |
| GV/SLV (%) | 48.8 ± 9.9 | 32.5 ± 2.0 | <0.001 |
| GRWR (%) | 0.98 ± 0.26 | 0.62 ± 0.06 | <0.01 |
| Left lobe/right lobe/others | 38/16/1 | 4/0/2 | 0.56 |
| Operation | |||
| Operation time (min) | 947 ± 252 | 1078 ± 233 | 0.23 |
| Blood loss (mL) | 6836 ± 12,023 | 10,885 ± 9918 | 0.36 |
GRWR = graft-to-recipient body-weight ratio; GV/SLV = graft volume divided by standard liver volume; HCC = hepatocellular carcinoma; MELD = model for end-stage liver disease.
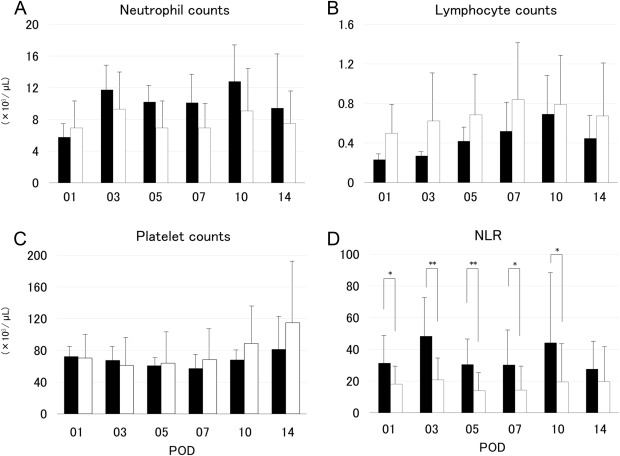
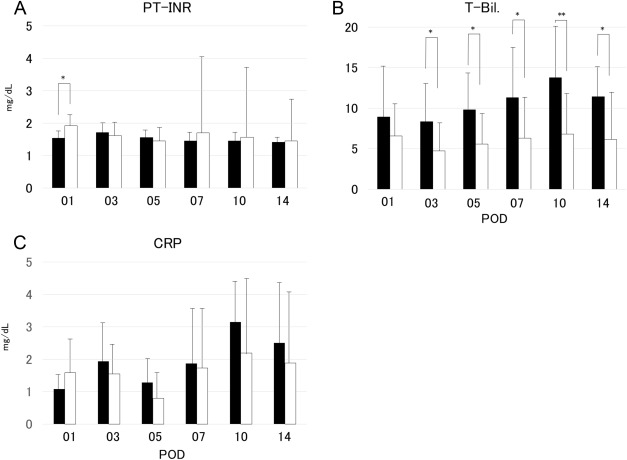
The neutrophil and lymphocyte absolute counts in Groups L and S did not differ significantly (Figures 1A and 1B); further, the platelet counts also showed no significant differences (Figure 1C). However, the NLR showed significant differences between the two groups from POD 3 to POD 10 (Figure 1D). The post-transplantation prothrombin time–international normalized ratio was not significantly different between the two groups, except on POD 1 (Figure 2A). By contrast, the total bilirubin levels differed significantly between the two groups from POD 3 to POD 14 (Figure 2B). The C-reactive protein in Groups L and S did not differ significantly (Figure 2C).
|
|
|
Figure 1. Changes in the absolute numbers of (A) neutrophils, (B) lymphocytes, (C) platelets, and (D) neutrophil-to-lymphocyte ratio in the recipients using graft volume/standard liver volume under 35% graft group (Group S; closed bars) and in those using graft volume/standard liver volume over 35% graft group (Group L; open bars). The differences in neutrophil-to-lymphocyte ratio between the two groups were significant at postoperative Day 1, Day 7, and Day 10 (p < 0.05), as well as at postoperative Day 3 and Day 5 (p < 0.01). NLR = neutrophil-to-lymphocyte ratio; POD = postoperative day. |
|
|
|
Figure 2. Changes in (A) prothrombin time–international normalized ratio, (B) serum total bilirubin level, and (C) C-reactive protein in recipients using graft volume/standard liver volume under 35% graft group (Group S; closed bars) and in those using graft volume/standard liver volume over 35% graft group (Group L; open bars). The difference in prothrombin time–international normalized ratio between the two groups was significant at postoperative Day 1 (p < 0.05). Further, the differences in the total bilirubin levels between the two groups were significant at postoperative Day 3, Day 5, Day 7, and Day 14 (p < 0.05), as well as at postoperative Day 10 (p < 0.01). CRP = C-reactive protein; POD = postoperative day; PT–INR = prothrombin time–international normalized ratio; T-Bil. = total bilirubin. |
According to the postoperative clinical course, the incidence of post-transplantation complications (i.e., prolonged hyperbilirubinemia and SFS graft syndrome) differed significantly between the two groups. However, the incidence of other postoperative complications, including acute cellular rejection, infection, graft loss, and relaparotomy because of postoperative bleeding, bowel perforation, or vascular complication, was not statistically different between the groups (Table 2).
| Factors | Group L (n = 55) | Group S (n = 6) | p |
|---|---|---|---|
| Infections | 8 (14.5) | 1 (16.7) | 0.64 |
| Acute cellular rejection | 12 (21.8) | 0 (0.0) | 0.46 |
| Bilirubin on POD 14 > 10 mg/dL | 10 (18.2) | 4 (66.7) | <0.05 |
| Ascites on POD 14 > 1000 mL | 13 (23.6) | 1 (16.7) | 0.90 |
| PT–INR on POD 14 > 1.5 | 11 (20.0) | 2 (33.3) | 0.82 |
| Small-for-size graft syndrome | 0 (0.0) | 2 (33.3) | <0.01 |
| Biliary complications | 10 (18.2) | 0 (0.0) | 0.57 |
| Vascular complications | 7 (12.7) | 0 (0.0) | 0.80 |
| Relaparotomy during postoperative hospital stay | 16 (29.1) | 4 (66.7) | 0.16 |
| Graft loss within 1 year postoperatively | 10 (18.2) | 3 (50.0) | 0.20 |
Data are presented as n (%).
POD = postoperative day; PT–INR = prothrombin time–international normalized ratio.
4. Discussion
LDLT is an important therapeutic modality for patients suffering from end-stage liver disease. However, following LDLT, problems related to the SFS graft syndrome remain unresolved. Many reports have described various issues related to graft size in LDLT,1; 2 ; 3 although elucidating these specific postoperative findings has been still difficult.
One of the most important factors related to the SFS graft-associated symptoms has been reported to be sinusoidal endothelial injury due to various reasons, such as ischemia/reperfusion, usage of immunosuppressant agents, and excessive portal vein pressure.17 Endothelial injury is reported to cause extensive inflammatory changes, which strongly affect the blood-coagulation system.18 Coagulopathies cause microcirculatory disturbances in the graft and impaired graft function. In this report, we used NLR to evaluate the severity of inflammation in LDLT recipients, demonstrating statistically significant differences in the early postoperative period in the small graft group.
NLR has been reported to have a relationship with the clinical course in various diseases, such as solid tumors (including HCC before liver transplantation) and acute coronary syndrome.7; 8; 9; 10; 11 ; 12 In patients with myocardial infarction, the elevation of NLR might be related to impaired microvascular perfusion due to rheological changes. In our study, pathophysiological changes concerning post-transplantation NLR are suggested to have a resemblance to these vascular injuries, because the graft is exposed to similar ischemia/reperfusion injuries to acute coronary syndrome, and also, excessive shear stress for sinusoidal endothelial cell, which is unavoidable in LDLT using SFS graft. Thus, we speculate that the mechanism of NLR elevation is due to the induction of strong inflammatory changes by these vascular injuries.
In the domain of vascular diseases, neutrophilia is itself a prognostic factor.19 Therefore, neutrophil counts are suggested to have a strong relationship with vascular endothelial cell injury. We have previously reported that the neutrophil elastase inhibitor (sivelestat) has a suppressive effect on hepatic ischemia/reperfusion injury, suggesting the effectiveness and importance of suppressing neutrophil activation.20 By contrast, excessive and persistent suppression of neutrophils can lead to infections; thus, appropriate suppression is considered important in the clinical setting.
The relationship between inflammatory changes of the endothelium and activation of neutrophils and platelets is very important.21 As suggested in the present study, neutrophil activation is an important pathophysiology of graft sinusoidal endothelial injury in LDLT. In damaged sinusoids, activated neutrophils and platelets migrate to the space of Disse. Moreover, platelet aggregation is observed in this condition; we named this phenomenon “extravasated platelet aggregation.”22 As mentioned previously, various transplant-related pathophysiologies can cause sinusoidal endothelial injury, which induces extravasated platelet aggregation and sequential graft dysfunction.
For LDLT using SFS graft, splenectomy is the most common surgical method of inflow modulation. Its beneficial effects are considered mostly to be due to reduced portal venous flow to the smaller vascular bed.23 However, it is possible that other mechanisms are involved in the beneficial effects of splenectomy, as suggested by the influence of lymphocytic functionality and differentiation.24 In addition, beneficial immunological modulation by portal venous infusion of prostaglandin E1 (PGE1) therapy applied in ABO-incompatible transplantation has been reported.25 PGE1 acts not only on portal venous pressure decompression, but also by immunological modulatory functions. Further, PGE1 is suggested to protect graft microcirculation via endothelial cytoprotection by cyclic adenosine monophosphate.26 Thus, our present results support speculations about relationships between graft size, sinusoidal endothelial injury, immunological reaction, graft microcirculation, and postoperative clinical course.
The effects of small intestinal congestion induced by portal venous hypertension should not be ignored in the pathophysiology of the SFS graft syndrome. Congestion of the small intestine has been reported to induce mucosal apoptosis in an experimental model.27 Such intestinal injury via tumor necrosis factor-alpha leads to inflammation and immunological activation, which affect the neutrophil activation and lymphocyte interactions.28 Further, preserving the small intestinal immunity is important in the setting of portal venous congestion induced by SFS grafts in LDLT.
The small number of presented cases is a limitation of this retrospective study. The small sample size affected statistical power of this study, because postoperative course of LDLT recipient affected various pathophysiological factors. However, NLR is an easily collected and measured biomarker. Moreover, the elevation of the NLR in the smaller graft group reflects a suggestive pathophysiology of endothelial injuries that related to SFS graft syndrome in LDLT. Further studies are required to establish its utility, and enhance our understanding of the relationship between graft size and elevation of the NLR.
Acknowledgments
The authors would like to express the deepest appreciation to Dr Takashi Tani (Public Central Hospital of Matto Ishikawa) and Dr Koichi Shimizu (Toyama Prefectural Central Hospital) for their sincere encouragement and insightful support to our transplant program team.
References
- 1 T. Yoshizumi, A. Taketomi, H. Uchiyama, et al.; Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation; Liver Transpl, 14 (2008), pp. 1007–1013
- 2 M. Taniguchi, T. Shimamura, S. Todo, H. Furukawa; Small-for-size syndrome in living-donor liver transplantation using a left lobe graft; Surg Today, 45 (2015), pp. 663–671
- 3 Y. Soejima, M. Shimada, T. Suehiro, et al.; Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe; Liver Transpl, 9 (2003), pp. 581–586
- 4 M. Lesurtel, D.A. Raptis, E. Melloul, et al.; Low platelet counts after liver transplantation predict early posttransplant survival: the 60-5 criterion; Liver Transpl, 20 (2014), pp. 147–155
- 5 G.J. Guthrie, K.A. Charles, C.S. Roxburgh, P.G. Horgan, D.C. McMillan, S.J. Clarke; The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer; Crit Rev Oncol Hematol, 88 (2013), pp. 218–230
- 6 J. Shindoh, Y. Sugawara, R. Nagata, et al.; Evaluation methods for pretransplant oncologic markers and their prognostic impacts in patient undergoing living donor liver transplantation for hepatocellular carcinoma; Transpl Int, 27 (2014), pp. 391–398
- 7 S.R. Walsh, E.J. Cook, F. Goulder, T.A. Justin, N.J. Keeling; Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer; J Surg Oncol, 91 (2005), pp. 181–184
- 8 K.M. Sarraf, E. Belcher, E. Raevsky, A.G. Nicholson, P. Goldstraw, E. Lim; Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small lung cancer; J Thorac Cardiovasc Surg, 137 (2009), pp. 425–428
- 9 H. Cho, H.W. Hur, S.W. Kim, et al.; Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment; Cancer Immunol Immunother, 58 (2009), pp. 15–23
- 10 D. Gomez, S. Farid, H.Z. Malik, et al.; Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma; World J Surg, 32 (2008), pp. 1757–1762
- 11 B.K. Duffy, H.S. Gurm, V. Rajagopal, R. Gupta, S.G. Ellis, D.L. Bhatt; Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention; Am J Cardiol, 97 (2006), pp. 993–996
- 12 P.H. Gibson, B.L. Croal, B.H. Cuthbertson, et al.; Preoperative neutrophil–lymphocyte ratio and outcome from coronary artery bypass grafting; Am Heart J, 154 (2007), pp. 995–1002
- 13 K. Urata, S. Kawasaki, H. Matsunami, et al.; Calculation of child and adult standard liver volume for liver transplantation; Hepatology, 21 (1995), pp. 1317–1321
- 14 H. Hayashi, H. Takamura, T. Tani, et al.; Partial portal arterialization for hepatic arterial thrombosis after living-donor liver transplant; Exp Clin Transplant, 10 (2012), pp. 247–251
- 15 P.S. Kamath, R.H. Wiesner, M. Malinchoc, et al.; A model to predict survival in patients with end-stage liver disease; Hepatology, 33 (2001), pp. 464–470
- 16 D. Dindo, N. Demartines, P.A. Clavien; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey; Ann Surg, 240 (2004), pp. 205–213
- 17 D. Palmes, T.B. Budny, K.H. Dietl, H. Herbst, U. Stratmann, H.U. Spiegel; Detrimental effect of sinusoidal overperfusion after liver resection and partial liver transplantation; Transpl Int, 17 (2005), pp. 862–871
- 18 S. Gando; Microvascular thrombosis and multiple organ dysfunction syndrome; Crit Care Med, 38 (2010), pp. S35–42
- 19 I. Karabinos, S. Koulouris, A. Kranidis, S. Pastromas, N. Exadaktylos, A. Kalofoutis; Neutrophil count on admission predicts major in-hospital events in patients with a non-ST-segment elevation acute coronary syndrome; Clin Cardiol, 32 (2009), pp. 561–568
- 20 S. Sakai, H. Tajima, T. Miyashita, et al.; Sivelestat sodium hydrate inhibits neutrophil migration to the vessel wall and suppresses hepatic ischemia–reperfusion injury; Dig Dis Sci, 59 (2014), pp. 787–794
- 21 K. Akinosoglou, D. Alexopoulos; Use of antiplatelet agents in sepsis: a glimpse into the future; Thromb Res, 133 (2014), pp. 131–138
- 22 S. Nakanuma, T. Ohta, H. Tajima, et al.; Extravasated platelets aggregation in liver zone 3 may correlate with the progression of sinusoidal obstruction syndrome after living donor liver transplantation: a case report; Exp Ther Med, 9 (2015), pp. 1119–1124
- 23 V. Raut, R. Alikhanov, J. Belghiti, S. Uemoto; Review of the surgical approach to prevent small-for-size syndrome in recipients after left lobe adult LDLT; Surg Today, 44 (2014), pp. 1189–1196
- 24 Y. Nomura, M. Kage, T. Ogata, et al.; Influence of splenectomy in patients with liver cirrhosis and hypersplenism; Hepatol Res, 44 (2014), pp. E100–109
- 25 T. Onoe, Y. Tanaka, K. Ide, et al.; Attenuation of portal hypertension by continuous portal infusion of PGE1 and immunologic impact in adult-to-adult living-donor liver transplantation; Transplantation, 95 (2013), pp. 1521–1527
- 26 H.M. Peshavariya, G.S. Liu, C.W. Chang, F. Jiang, E.C. Chan, G.J. Dusting; Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis; Antioxid Redox Signal, 20 (2014), pp. 2710–2725
- 27 B. Wu, T. Fujise, R. Iwakiri, et al.; Venous congestion induces mucosal apoptosis via tumor necrosis factor-alpha-mediated cell death in the rat small intestine; J Gastroenterol, 39 (2004), pp. 1056–1062
- 28 R. Klingenberg, T.F. Luscher; Inflammation in coronary artery disease and acute myocardial infarction—is the stage set for novel therapies?; Curr Pharm Des, 18 (2012), pp. 4358–4369
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?