Abstract
Background
Pulmonary vein isolation (PVI) for atrial fibrillation (AF) is performed with the endoscopically assisted laser balloon ablation system (EAS). We hypothesized that placement of a circular mapping catheter (CMC) in the pulmonary vein (PV) distal to the laser balloon during ablation is feasible and safe.
Methods
Out of 58 included patients, 37 underwent mapping-guided EAS PVI, with the CMC inside the PV during laser ablation, and 21 patients underwent standard EAS PVI, with the CMC outside the PV during laser ablation.
Results
Mean age was 56 years and 81% had paroxysmal AF. In the mapping-guided ablation group, 91% of PVs were isolated with the CMC in the PV during EAS ablation, isolation was completed in 9% of PVs after the CMC was removed from the PV. After passing a learning curve in 18 patients, a significant drop in unsuccessfully isolated PVs was observed in the mapping guided EAS PVI group (15% to 4%, P = 0.020). No major complications were seen in the mapping-guided EAS PVI group. However, in the standard EAS PVI group, laser ablation was complicated by a temporary phrenic nerve palsy in 1 patient. After a median follow-up of 16.7 months, there was no statistical difference in AF free survival among treatment groups (mapping-guided: 56% vs. 52%, P = 0.875).
Conclusion
Mapping guided EAS PVI with a distal CMC in the PV during laser ablation is feasible and seems safe as the standard EAS PVI approach.
Keywords
Atrial fibrillation;Ablation;Pulmonary vein isolation;Laser balloon;CMC
1. Introduction
The endoscopic laser balloon ablation system (EAS) [1]; [2]; [3] ; [4] is a technique that is being used to perform pulmonary vein isolation (PVI) in the treatment of atrial fibrillation (AF) [4]; [5] ; [6]. The EAS consists of a flexible, compliant balloon for sustained wall contact and a power adjustable laser beam for ablation independent of tissue contact. Despite visual guidance, with a standard EAS approach, electrical disconnection of the PVs from the left atrium could only be achieved in 70–80% of pulmonary veins (PVs) [7]. Postablation electrical mapping with a circular mapping catheter (CMC) therefore remains necessary. When electrical conduction persists after laser ablation, the CMC is inserted in the PV, followed by insertion of the EAS to close the gaps in the circumferential ablation lines [4]. However, there is no data available on the efficacy and safety of CMC-guided EAS PVI. The present study aims to report feasibility and safety aspects of mapping-guided EAS ablation with the CMC inside the PV during laser balloon ablation compared to standard EAS ablation.
2. Methods
Fifty-eight consecutive patients who underwent a primo PVI using the EAS between December 2011 and November 2013 were reviewed and patient data was collected from a prospective registry. Patients were randomly divided among two different approaches: the mapping-guided approach group, in which the CMC was positioned in the PV during ablation and the standard ablation group in which the CMC was outside the PV during ablation. The prospective registry has been approved by the Institutional Review Board. All patients underwent routine transoesophageal echocardiography prior to the procedure to rule out left atrial appendage thrombus. Furthermore, in accordance with local guidelines, patients stopped using anticoagulants and were ‘bridged’ with low molecular weight heparin in a weight-dependent dose until the day of ablation.
2.1. The endoscopic laser balloon
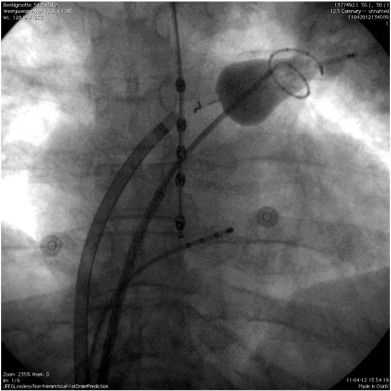
The EAS (CardioFocus, Marlborough, MA, USA) is a non-steerable, compliant balloon catheter. Its characteristics have been described previously as well as the pre-ablation protocol [8]. In summary, both the EAS and CMC (Lasso® Biosense Webster Inc., Diamond Bar, CA, USA) were transeptally inserted in the left atrium under fluoroscopic guidance. An activated clotting time of 300–350 s was maintained during the procedure using intravenous heparin. Before ablation, PV angiography was performed in all patients. An oesophageal temperature probe (SensiTherm, St. Jude Medical, USA) was inserted (Fig. 1), and energy delivery was instantaneously terminated when temperature exceeded 39.0 °C. During ablation of the right sided PVs, stimulation of the phrenic nerve (using 20 mA at 2.9 ms) was performed, with immediate cessation of energy delivery once capture was diminished or lost.
|
|
|
Fig. 1. Fluoroscopic image of the setup of the endoscopic laser balloon system (PA view). The CMC was inserted transseptally into the left atrium and placed in the left superior pulmonary vein (LSPV). The laser balloon catheter was also inserted transseptally into the left atrium and positioned in the LSPV proximal to the CMC. The temperature probe was positioned in the oesophagus. The quadripolar catheter was positioned in the coronary sinus. |
2.2. Mapping-guided ablation
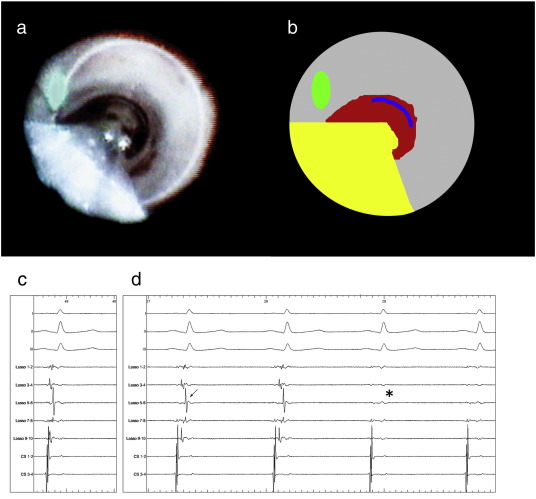
In the mapping-guided approach group, the CMC was first positioned into the PV. Previously recorded templates of PV potentials were used to assure correct placement of the CMC. Secondly, the EAS catheter was inflated in the same PV proximal to the CMC (Fig. 1). During inflation the blind spot of the EAS was positioned over the shaft of the CMC (Fig. 2). Thereafter, an almost complete circular ablation was performed while observing the PV potentials of the mapping catheter. The ablation circle was completed by rotating the EAS and the CMC to expose the previously obscured atrial tissue, i.e. ‘blind-spot’. A full circle was always performed, also when PVI occurred during ablation. When, after a full ablation circle, electrical connection between the LA and the PV still persisted, gap ablation was performed, as described by previous reports [4]. When a PV was insufficiently occluded for circular ablation because of interference of the CMC, the CMC was removed from the PV, and an ablation circle was completed.
|
|
|
Fig. 2. Panels a and b. Panel a displays the endoscopic view from the EAS in the left superior pulmonary vein (LSPV) with the CMC in the PV, viewing directly into the PV. First, the CMC was positioned in the PV. Then, the EAS was positioned proximally in the same PV and inflated until a white ring of atrial tissue (PV antrum) was visualized, indicating fixation of the laser balloon and optimal balloon-tissue contact. The EAS catheter shaft was positioned over the CMCs shaft. Panel b shows the EAS shaft (yellow), laser aiming beam (green), exposed PV antrum (grey) blood distally in the PV (red) and a part of the CMC (blue). Panels c and d. Electrical signals before (c) and during (d) laser ablation with the CMC (Lasso, Biosense Webster, USA) positioned distal to the laser balloon in the LSPV. The top three electrograms are standard ECG leads (I, III and V1), the middle 5 are PV potentials recorded with the CMC, the two coronary sinus bipolar electrograms are at the bottom. In panel c, the electrical potentials before laser balloon ablation are shown. The top three electrograms are standard ECG leads (I, III and V1). The bipolar PV potentials recorded with decapolar CMC are shown. The LSPV prior to ablation depicted large PV potentials (arrows). In panel D the change in electrical potentials during PV isolation are depicted. Note the conduction delay at the veno-atrial junction compared to the template in panel c (arrows), followed by conduction block (asterisk). |
2.3. Standard laser balloon ablation
In the standard approach, the EAS was manoeuvred to each PV ostium. PV occlusion was attempted by varying balloon inflation size until a ring of atrial myocardium antral to the PV was exposed. Laser energy was delivered to the exposed ring of atrial tissue (Fig. 2). The ablation circle was completed by rotating the EAS to expose the previously obscured atrial tissue, i.e. ‘blind-spot’. After a full circle was completed, the EAS was retracted from the PV. The CMC was introduced to assess persistent electrical connection between the PV and the LA. If any existed, the EAS was re-introduced to the PV, and additional lesions were applied by the operator to close gaps in the PV antral ablation lines. No adenosine testing was performed.
2.4. Follow-up
Patients visited the outpatient clinic at 3, 6, 12 and 24 months after PVI, including 24-hour Holter ECG. AF recurrence was defined in accordance with current European AF ablation guidelines [9]. In case of symptoms, patients were immediately referred to acquire an ECG. 3 months after PVI, an attempt was made in all patients to cease AADs.
2.5. Study endpoints
The primary endpoint of our study was acute PVI using the mapping-guided or the standard approach. Secondary endpoints were procedure time, ablation time and fluoroscopy time. Complications were defined in accordance with European guidelines [9].
2.6. Statistical analysis
Patients were appointed by the type of procedure (mapping-guided approach versus standard). Continuous variables were expressed as mean with standard deviation in case of normal distribution. Significance in differences was analysed by the Mann–Whitney U test. Categorical data were expressed as numbers and percentages and compared with Chi-square test. The difference in AF free survival was assessed with a log-rank test. Statistical analysis was performed using IBM SPSS statistics version 20 (IBM Inc., Armonk, NY, USA). A P-value of ≤ 0.05 was considered statistically significant. The artwork in Fig. 2 was created using Adobe Photoshop CS2 (Adobe Inc., San Jose, CA, USA).
3. Results
Fifty-eight consecutive patients were included, mapping-guided PVI was performed in 37 patients. Baseline characteristics were comparable between treatment groups, as displayed in Table 1.
| Mapping-guided approach (n = 37) | Standard (n = 21) | P-value | Total group (n = 58) | |
|---|---|---|---|---|
| Age (years) | 54.8 ± 10.1 | 58.7 ± 11.8 | 0.178 | 56.2 ± 10.8 |
| Sex Female (%) | 9 (24%) | 7 (33%) | 0.461 | 16 (28%) |
| BMI (kg/m2) | 27.2 ± 4.0 | 26.5 ± 3.3 | 0.508 | 26.9 ± 3.6 |
| Non-paroxysmal AF | 7 (19%) | 4 (14%) | 0.990 | 11 (19%) |
| AF duration (years) | 6.1 (± 5.7) | 7.4 (± 7.1) | 0.459 | 6.6 ± 6.2 |
| Hypertension | 27 (73%) | 11(52%) | 0.113 | 38 (66%) |
| Diabetes | 1 (3%) | 2 (10%) | 0.260 | 3 (5%) |
| Failed AADs | 1.4 (0–4) | 1.4 (0–3) | 0.952 | 1.4 (0–4) |
| LA dimension in PSLAX (mm) | 41.1 (± 4.0) | 41.1 (± 4.0) | 0.984 | 41.1 (± 3.9) |
| LVEF (%) | 58.4 (± 3.6) | 59.4 (± 2.4) | 0.239 | 58.7 (± 3.3) |
Data are presented as absolute numbers or means with ± their SD, with percentages or ranges where appropriate. P-values are between mapping-guided and standard approach. BMI denotes body mass index, AF; atrial fibrillation, AAD; anti-arrhythmic drugs, LA; left atrium, PSLAX; parasternal long axis view, LVEF; left ventricular ejection fraction.
3.1. Efficacy of PVI using the mapping-guided versus standard approach
In 148 PVs where isolation was attempted with the mapping-guided EAS PVI approach, isolation of 134 PVs (91%) was accomplished with the CMC being positioned inside the PV during ablation. Due to suboptimal PV occlusion with the endoscopic laser balloon, the CMC was removed in 14 PVs (10 patients) and all PVs were successfully isolated with the CMC outside the PV. There was no significant difference among the PVs with suboptimal occlusion (P = 0.623): 4 left superior PVs, 2 left inferior PVs, 3 right superior PVs and 5 right inferior PVs. Of note, in the first 18 patients in the mapping guided EAS PVI group, 11 PVs could not be isolated with the CMC in the PV, compared to 3 in the last 18 patients (P = 0.020). In patients treated with the standard EAS PVI approach, 84/85 PVs were isolated (98.8%). In 1 common left sided PV, isolation could not be achieved due to oesophageal temperature rise. One procedure in the standard approach group was converted to RF ablation, after right sided PV ablation was complicated by a temporary phrenic nerve palsy.
3.2. Procedural characteristics
There was no significant difference among treatment groups in terms of procedure duration and fluoroscopy time. However, ablation time was significantly shorter in the mapping-guided approach group compared to the standard approach group (51 vs. 71 min, P = 0.017). Table 2 displays the procedural characteristics among treatment groups.
| Mapping-guided approach (n = 37) | Standard approach (n = 21) | p-Value | Total group (n = 58) | |
|---|---|---|---|---|
| Procedure time (min) | 179 (± 42) | 170 (± 40) | 0.469 | 175 (± 41) |
| Ablation time (min) | 51 (± 25) | 71 (± 28) | 0.017 | 59 (± 28) |
| Fluoroscopy time (min) | 35 (± 9) | 34 (± 12) | 0.849 | 35 (± 10) |
Data are presented as means ± their SD. P-values are between mapping-guided and standard approach.
3.3. Safety
There were no complications among patients treated with the mapping-guided approach. In the standard ablation group, one patient suffered from a temporary phrenic nerve palsy. This patient has fully recovered after 6 months. There have been no patients suffering from thromboembolic events in either ablation approach.
3.4. Follow-up
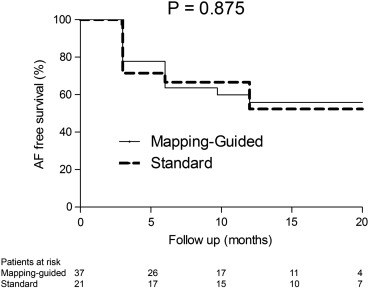
After a median follow-up of 16.7 (q1–q3: 11.2–19.9) months, 33 (57%) patients were still free of AF recurrences after a single EAS PVI attempt. None of the patients who were free of AF were using AADs at the end of the study period. AF free survival was 55.9% in the mapping-guided approach group and 52.3% in the standard approach group (P = 0.875), as displayed in Fig. 3.
|
|
|
Fig. 3. AF free survival after EAS PVI. This figure depicts the AF free survival after EAS PVI for both treatment groups. AF free survival is not significantly different among treatment groups, P = 0.875. |
4. Discussion
The present study indicates that it is feasible and safe to perform a circumferential PVI using the mapping-guided EAS PVI approach with a CMC inside the PV during EAS ablation, with a comparable AF free survival. Of note, ablation time was significantly reduced in the mapping-guided approach group. Nevertheless, in 9% of PVs, the CMC was removed from the PV during ablation due to suboptimal PV occlusion.
4.1. Pulmonary vein isolation
The present study showed that a mapping-guided EAS ablation resulted in an acutely isolated PV in 134 out of 148 attempted PVs. In 14 PVs, PV occlusion could not be achieved with the CMC in the PV during ablation, and the CMC needed to be removed from the PV. Of note, there seemed to be a learning curve present, and in the last 18 patients of the mapping guided EAS PVI group, only 3 PVs could not be isolated with the CMC in the PV. A learning curve was also observed in a series of 150 EAS patients [10].
4.2. Complications
There were no complications in the mapping-guided approach EAS PVI group, although a study with a larger patient population is necessary to demonstrate the safety of mapping-guided ablation. Of note, in order to prevent complications, it seems important to place the blind spot of the EAS over the shaft of the circular ablation catheter to prevent energy application on the CMC shaft itself, since this may result in overheating of the catheter shaft and thereby thrombus formation or balloon perforation.
4.3. Potential advantages
Witnessing conduction slowing and block of PV potentials during PVI is a definitive argument in achieving acute PVI. For example, in the standard EAS PVI approach, placement of the CMC too distally in the PV after removal of the EAS from the PV may result in erroneously identifying a PV as being isolated. Moreover, the obviation of removal and re-introduction of the EAS during gap mapping avoids the possibility of applying ablation circles at different depths at the PV antrum. Potentially, multiple circular ablation lesions at different depths at the PV antrum in the standard approach can result in insufficiently overlapping ablation lesions and thereby influence AF free survival. Furthermore, the ablation time was significantly shorter in the mapping-guided approach group. Although this study was not aimed at identifying the mechanism for this difference, potentially, ablation with the mapping-guided approach is more efficient and reduces the need for gap ablations.
4.4. Future perspectives
This feasibility study with preliminary data shows that EAS ablation can be performed with the mapping-guided approach. Furthermore, we did not observe any complications related to the mapping-guided approach, suggesting a favourable safety profile, although the data is preliminary. The EAS is a relatively novel technology which has been proven to be effective and safe. There is a clear clinical need for an endoscopic laser balloon with PV mapping capabilities. Potentially, an adjusted endoscopic laser balloon with incorporation of mapping electrodes can result in reduction of procedure times and increased long-term efficacy.
4.5. Limitations
With regards to interpreting our data, the following limitations should be considered. Patients were not randomized to the different ablation procedures. Instead, patients were randomly allocated to PVI approaches. Current study consists of a limited sample size. In 10 patients from the mapping-guided approach group, a standard ablation approach was performed in 1 or 2 PVs. Excluding these patients from analysis did not affect the primary endpoint.
5. Conclusion
In conclusion, mapping-guided EAS PVI with the laser balloon is a feasible and safe technique. This study did not show an increase in complications, although larger studies are necessary to confirm safety and outcome issues.
Conflict of interest
None declared.
Funding
None.
Acknowledgements
We would like to thank Ms. Vera Derks for excellent editorial assistance.
References
- [1] V.Y. Reddy, P. Neuzil, S. Themistoclakis, S.B. Danik, A. Bonso, A. Rossillo, et al.; Visually-guided balloon catheter ablation of atrial fibrillation: experimental feasibility and first-in-human multicenter clinical outcome; Circulation, 120 (2009), pp. 12–20
- [2] S.R. Dukkipati, K.H. Kuck, P. Neuzil, I. Woollett, J. Kautzner, H.T. McElderry, et al.; Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience; Circ Arrhythm Electrophysiol, 6 (2013), pp. 467–472
- [3] S.R. Dukkipati, P. Neuzil, J. Skoda, J. Petru, A. d'Avila, S.K. Doshi, et al.; Visual balloon-guided point-by-point ablation: reliable, reproducible, and persistent pulmonary vein isolation; Circ Arrhythm Electrophysiol, 3 (2010), pp. 266–273
- [4] B. Schmidt, A. Metzner, K.R. Chun, D. Leftheriotis, Y. Yoshiga, A. Fuernkranz, et al.; Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system; Circ Arrhythm Electrophysiol, 3 (2010), pp. 481–488
- [5] S. Bordignon, K.J. Chun, M. Gunawardene, A. Fuernkranz, V. Urban, B. Schulte-Hahn, et al.; Comparison of balloon catheter ablation technologies for pulmonary vein isolation: the laser versus cryo study; J Cardiovasc Electrophysiol, 24 (2013), pp. 987–994
- [6] S. Bordignon, K.R. Chun, M. Gunawardene, V. Urban, M. Kulikoglu, K. Miehm, et al.; Energy titration strategies with the endoscopic ablation system: lessons from the high-dose vs low-dose laser ablation study; Europace, 15 (2013), pp. 685–689
- [7] B. Schmidt, M. Gunawardene, V. Urban, M. Kulikoglu, B. Schulte-Hahn, B. Nowak, et al.; Visually guided sequential pulmonary vein isolation: insights into techniques and predictors of acute success; J Cardiovasc Electrophysiol, 23 (2012), pp. 576–582
- [8] P. Gal, J.J. Smit, A. Adiyaman, A.R. Ramdat Misier, P.P. Delnoy, A. Elvan; First dutch experience with the endoscopic laser balloon ablation system for the treatment of atrial fibrillation; Neth Heart J, 23 (2015), pp. 96–99
- [9] H. Calkins, K.H. Kuck, R. Cappato, J. Brugada, A.J. Camm, S.A. Chen, et al.; 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design; Europace, 14 (2012), pp. 528–606
- [10] L. Perrotta, S. Bordignon, D. Dugo, A. Furnkranz, K.J. Chun, B. Schmidt; How to learn pulmonary vein isolation with a novel ablation device: learning curve effects using the endoscopic ablation system; J Cardiovasc Electrophysiol, 25 (2014), pp. 1293–1298
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?