Summary
Background
The proinflammatory cytokines and growth-promoting factor are essential components of the wound healing process. We hypothesized that under healthy conditions, faster healing of intestinal anastomotic wound is due to an early upregulation of proinflammatory cytokines, cytokine-induced neutrophil chemoattractant-1 (CINC-1) that is followed by a quicker upregulation of homeostatic chemokine, monocyte chemoattractant protein-1 (MCP-1) and late upregulation of transforming growth factor (TGF-β).
Methods
We characterized the time course of CINC-1, MCP-1 and TGF-β release at four wounds (skin, muscle, small bowel, and colonic anastomosis) after surgery on 38 juvenile male Sprague Dawley rats. The tissue samples of each site were harvested at 0 (control), 1, 3, 5, 7 and 14 days postoperatively (n = 6–8/group) and analyzed by ELISA kits for CINC-1, MCP-1 and TGF-β.
Results
CINC-1 expression peaked earlier in muscle and colonic wounds when compared to skin and small bowel. MCP-1 levels were elevated early in skin and muscle wounds, but later expression of MCP-1 was shown in colonic wounds. TGF-β levels were unchanged in all wound sites.
Conclusion
An earlier peak in CINC-1 levels and later expression of MCP-1 were seen in colonic wounds, but no significant increase in TGF-β levels was observed. These findings support the early healing process in intestinal anastomotic wounds.
Keywords
CINC-1;intestinal anastomosis;MCP-1;TGF-β;wound
1. Introduction
The most common complication following colorectal surgery is anastomotic leakage, which is associated with a high mortality rate.1 ; 2 Wound healing is a complex procedure that requires a highly regulated series of events, including interactions between many cell types, production of different soluble factors, and growth of matrix components. Normal wound healing has four phases—hemostasis, inflammation, tissue formation, and tissue remodeling.3 The primary goal of wound repair is to achieve hemostasis, which represents the first step in wound healing.
The inflammatory phase begins after 6 hours of tissue injury. This phase is associated with some important events, such as migration of activated neutrophils, macrophages, and lymphocytes from the circulation into the injured tissue. Neutrophils are the first leukocytes to infiltrate the injured tissue, followed by monocytes.4 Engelhardt et al5 reported that neutrophil infiltration into a human skin wound is extremely high during the initial days, reaching a maximum level on Day 1. Neutrophils are dominant in the wound site during the initial days. By contrast, macrophages (activated monocytes) reached their maximum level on Day 2. Chemokines selectively mediate the recruitment of neutrophils and monocytes in the affected area. Interleukin-8 (IL-8) mRNA expression in the wound site is maximum on Day 1,6 and then it declines to reach its lowest level on Day 4. Another neutrophil chemokine, growth-regulated oncogene (GRO-α), is also expressed within 4 days after wounding. There is a strong correlation between the levels of IL-8, GRO-α, and neutrophil infiltration. The cooperative expression of IL-8 and GRO-α in the wound site enhances neutrophil migration to this site because each of them stimulates neutrophils via different receptors. Importantly, rats lack a homolog of human IL-8, but they express a cytokine with similar properties and as potent a chemoattractant as IL-8, named cytokine-induced neutrophil chemoattractant (CINC-1).7; 8 ; 9
Monocytes infiltrate the injured tissue in response to chemotactic cytokines such as monocyte chemoattractant protein-1 (MCP-1). MCP-1 is released from basal keratinocytes adjacent to the epidermis or from endothelial cells.6 Peak levels of MCP-1 are detected 1–2 days after skin wounding and slowly decline thereafter to Day 7.5 Transforming growth factor-α (TGF-α) is another important cytokine for extracellular matrix synthesis and remodeling, which is released by monocytes and macrophages. These growth factors and cytokines function to promote the last two phases of healing: tissue formation and tissue remodeling.10 Thus, an alteration of the expression or function of inflammatory cytokines may lead to the persistence of an inflammatory phase and alter the normal wound healing process. Therefore, successful acute wound healing occurs when proinflammatory cytokines, such as CINC-1, control the recruitment of neutrophils in inflammatory and tissue injury phases, and homeostatic chemokines, such as MCP-1, fulfill the housekeeping functions in cleaning the wound from debris during the hemostasis phase.11
Based on these observations, we hypothesized that under healthy conditions, faster healing of intestinal anastomotic wound in comparison to skin and muscle incisional wound is due to early upregulation of proinflammatory cytokine (CINC-1), followed by quicker upregulation of homeostatic chemokine (MCP-1) and late upregulation of TGF-β. To the best of our knowledge, this is the first study to investigate temporal expressions of CINC-1 and MCP-1 at four different tissues in rats: skin, muscle, small intestine, and colon.
2. Materials and methods
2.1. Animals
Thirty-eight male Sprague–Dawley rats (200–250 g) (animals were obtained from animal house of College of Medicine, King Saud University) were used for the study. Each rat was housed individually in a single cage and placed in a room with controlled temperature and light/dark cycle (12 h/12 h). The animals were allowed ad libitum intake of normal rat pellet. Thirty-eight animals, divided into groups with six rats in each group, underwent surgery; enzyme-linked immunosorbent assay (ELISA) was performed for CINC-1, MCP-1, and TGF-β at the following time points: 0 (control), Day 1, Day 3, Day 5, Day 7, and Day 14. Studies were conducted according to a protocol approved by the Animal Care Committee of the College of Medicine, King Saud University, Riyadh, Saudi Arabia.
2.2. Surgical procedure
The animals were anesthetized with 1–2% inhalational halothane. A single preoperative prophylactic dose of cefazolin (30 mg/kg; Novopharm Limited, Toronto, Ontario, Canada) was administered subcutaneously. The abdomen was prepared, shaved, and entered through a midline incision. Transection with immediate reanastomosis was performed both at the terminal ileum (10 cm proximal to the ileocecal valve) and at the transverse colon (1 cm proximal to the splenic flexure). The techniques were similar to previously published methods.12 ; 13 A standardized end-to-end anastomosis was performed with 8–10 interrupted inverting sutures using 6-0 monofilament polypropylene (Prolene TM; Ethicon, Parsippany, NJ, USA). Polypropylene was chosen because of its inert characteristics, minimizing the inflammatory reaction associated with resorption of an absorbable suture.12 ; 13 The abdomen was washed out with sterile normal saline and closed in layers using interrupted sutures of 4-0 polypropylene in the fascia and skin.
After the procedure, rats were housed in single cages. The animals were allowed water immediately and solid food at 24 hours postsurgery (20 g/d). Daily oral intake and weight were recorded. At the time of sacrifice, rats were anesthetized with halothane/oxygen using a nose cone. Midline cutaneous and fascial wounds were harvested for 5 mm on each side of the wound, dissecting the skin and fascia away from the underlying tissue, followed by an en bloc excision without disturbing the actual wound. A laparotomy was then performed. Both the small bowel and the colonic anastomoses were identified and dissected away gently from the surrounding tissues. Care was taken to avoid disrupting adhesions as much as possible. If the anastomosis was not intact, the animal was excluded from further study. A 5 mm segment from each side of each anastomosis was excised en bloc. The rats were then killed by an intracardiac injection of 50 mg/kg sodium pentobarbital. All harvested tissues were wrapped in an aluminum foil and frozen immediately in a liquid nitrogen bath. All frozen tissue samples were kept at −80°C until further processing for cytokine measurement.
2.3. Extraction of tissue proteins
Tissues were processed according to the method reported by Zubaidi et al13 and Takahashi and Das14 with some modification. In brief, each tissue specimen was thawed, minced into 2 mm cubes, washed five times in 5 vol. of phosphate buffered saline (PBS), pH 7.2, containing 2 mM phenylmethylsulfonyl fluoride and 2 mM sodium azide. After centrifugation at 1000 g for 5 minutes, the tissue pellet was homogenized in the same buffer including 1 mM EDTA [5 vol. (wt/vol.)] on ice in a Polytron (Kinematic PT, Dispersing and Mixing Technology; Lucerne, Switzerland) at 5–10× speed for four 10-second intervals. Homogenates were centrifuged at 2000 g for 20 minutes to remove large tissue particles and recentrifuged at 13,200 g for 90 minutes to obtain a clear supernatant. Supernatants of each tissue specimen were further divided in 200 μL aliquots, labeled, and stored at −70°C. Prior to each experiment, the extracts were thawed and centrifuged at 13,200g for 5 minutes to remove debris.
2.4. ELISA assays
The supernatants were assayed quantitatively for CINC-1, MCP-1, and TGF-β (Assay Design, Ann Arbor, MI, USA), using a 96-well microtiter plate reader. The respective cytokine levels of the different tissue samples were determined by the means of duplicated measurements requiring 50–200 μL of extract. Results were verified using the internal controls supplied with each kit, with a known concentration of the target protein. Optical density measurements were then verified against a standardized curve. The results were averaged at the end of the experiment and expressed in pg/g wet tissue weight.
2.5. Statistical analysis
Results of cytokine and growth factor measurements for all tissues were plotted on graphs against the time of sacrifice. One-way analysis of variance (ANOVA) was used to compare each cytokines or growth factors in the four different wound sites at the same time point. ANOVA repeated measures were used to compare the temporal expression of each factor in a specific wound over the period of the study. Post hoc testing was performed using the Bonferroni and Dunnett T3 for multiple comparison, with p < 0.05 being considered significant; SPSS version 19 (SPSS Inc., Chicago, IL, USA) was used to perform the analysis. Results were expressed as mean ± standard error of mean.
3. Results
All animals in this study were healthy, eating equivalent amounts of chow, and gaining weight over the study period. No visual evidence of dehiscence (abscess, fistula formation, or fecal contamination within the peritoneum) was reported at the time of the harvest in any of the animals. Detection of CINC-1, MCP-1, and TGF-β expressions using the ELISA technique was investigated at the selected time points (0, Day 1, Day 3, Day 5, Day 7, and Day 14). Results of the quantitative analyses differed considerably between the different wound samples and are reported separately for each wound tissue (skin, muscle, small bowel, and colon).
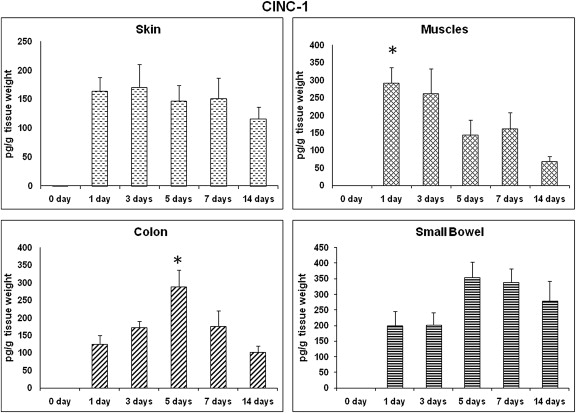
Initially, CINC-1 levels of skin and small bowel wound sites showed similar patterns of expression at all selected time points, except for the control that showed a significantly lower level (Fig. 1). On Day 1 postwounding, muscle CINC-1 levels increased dramatically compared to other selected time points but was significantly different from that on Day 14 (291.1 ± 107.7 pg/g wt vs. 69.5 ± 34.8 pg/g wt, respectively; p < 0.05; Fig. 1). By contrast, colon CINC-1 levels tended to be higher on Day 5 than on other time points but were not statistically significant except on Day 14, which showed significantly lower (p < 0.05) expression than that on Day 5 ( Fig. 1).
|
|
|
Figure 1. CINC-1: time course of expression. CINC-1 profile in different wound sites over time. CINC-1 levels from wound tissues were assessed using ELISA and are expressed as pg CINC-1/g wet tissue. Data: mean ± SEM, n = 6–8 for each wound site, at each time point. Statistical comparison over time by ANOVA (p < 0.05). ANOVA = analysis of variance; CINC = cytokine-induced neutrophil chemoattractant; ELISA = enzyme-linked immunosorbent assay; SEM = standard error of the mean. |
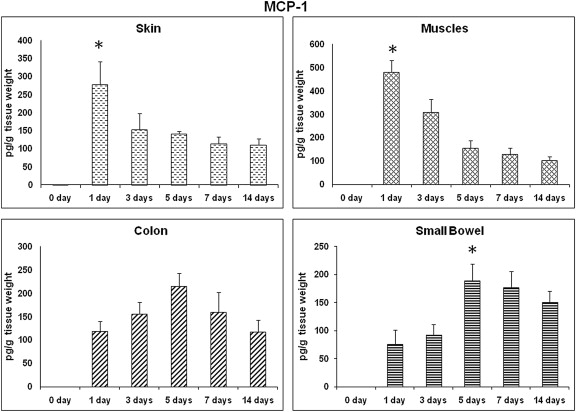
MCP-1 showed a distinct expression pattern in the small bowel and colon wound sites in comparison to the other wound sites. In these two wound sites, the expression of MCP-1 showed the normal expected distribution, i.e., a bell-shaped curve, throughout these time points (Fig. 2). For the small bowel wounds, MCP-1 levels were significantly higher on Day 5 in comparison to that on Day 1 (189.5 ± 70.9 pg/g wt vs. 75.7 ± 61.9 pg/g wt, respectively; p < 0.05). MCP-1 expression showed a similar pattern in colon wounds to that in the small bowel wounds; there was no significant difference in these MCP-1 expressions ( Fig. 2). By contrast, MCP-1 expression in skin and muscle wounds was completely opposite to that in small bowel and colon wounds, where it showed the highest level on Day 1 among all time points. Particularly in muscle wounds, MCP-1 expression was significantly higher on Day 1 and Day 3 than at other time points (p < 0.05; Fig. 2).
|
|
|
Figure 2. MCP-1: time course of expression. MCP-1 profile in different wound sites over time. MCP-1 levels from wound tissues were assessed using ELISA and are expressed as pg MCP-1/g wet tissue. Data: mean ± SEM, n = 6–8 for each wound site, at each time point. Statistical comparison over time by ANOVA (p < 0.05). ANOVA = analysis of variance; ELISA = enzyme-linked immunosorbent assay; MCP = monocyte chemoattractant protein; SEM = standard error of the mean. |
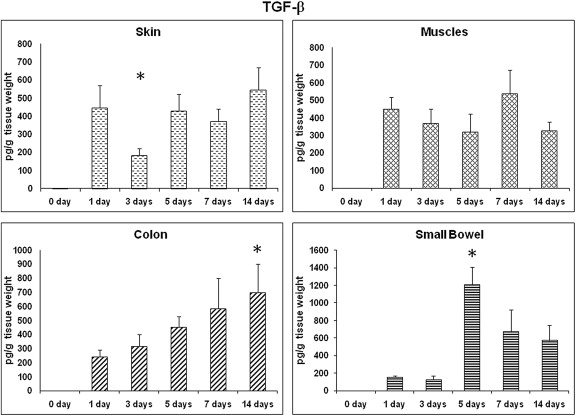
Fig. 3 shows TGF-β expressions in all different tissues at all the time points. In contrast to what has been shown in a previous study,13 the prohealing TGF-β showed a significant increase on Day 5 in the small bowel wounds. However, TGF-β levels showed no significant change in other wound sites.
|
|
|
Figure 3. TGF-β: time course of expression. TGF-β profile in different wound sites over time. TGF-β levels from wound tissues were assessed using ELISA and are expressed as pg TGF-β/g wet tissue. Data: mean ± SEM, n = 6–8 for each wound site, at each time point. Statistical comparison over time by ANOVA (p < 0.05). ANOVA = analysis of variance; ELISA = enzyme-linked immunosorbent assay; SEM = standard error of the mean; TGF = transforming growth factor. |
4. Discussion
Recruitment of inflammatory cells, neutrophils, and monocytes/macrophages to the wound sites for sterilization and production of growth factors is an essential process for wound healing.5 ; 15 The dynamics and roles of proinflammatory mediators, such as cytokines and chemokines, in several types of injured and postburn tissues have recently attracted the attention of researchers.16 ; 17 Persistent inflammation in gastric injuries and ulcer recurrence was attributed to the overexpression of proinflammatory cytokines, such as IL-1β and tumor necrosis factor (TNF-α), which have many biological effects on chemokine production and neutrophil activation.18; 19 ; 20 Several studies have shown that healing of intestinal wounds is faster than skin wounds,21 ; 22 which is attributed to the difference in the expression of cytokines and growth factors between these two wound sites.13 The aim of this study was to assess the cytokine profile in postwounded skin, muscle, small bowel, and colon in a rat model that can be used for the characterization of alterations in proinflammatory chemokines expression patterns at different time points—0, Day 1, Day 3, Day 5, Day 7, and Day 14.
Chemokines are low-molecular-weight (8–10 kd) chemotactic cytokines. They represent the chemotactic signals that are produced by different cell types to initiate leukocyte infiltration into inflamed or injured tissues. They perform these functions by stimulating and increasing the expression of adhesion molecules. Chemokines are divided into four families, according to the position of cysteine residues in these proteins: C, CC, CXC, and CX3C families. Two of them have extensively been characterized as the two largest chemokine families: α and β chemokines, corresponding to CXC and CC families, respectively.23 Among many chemoattractants, CINCs are known to have a potent chemotactic activity for neutrophils and the predominant chemokines in rats.24 CINCs share the greatest sequence homology with the human CXC chemokine GRO-α, which is a neutrophil chemotactic cytokine.25
As summarized in Fig. 1, our results showed that the levels of proinflammatory chemokine, CINC-1, were elevated significantly up to Day 3 in wounded muscle tissues and up to Day 5 in colonic wounds, as compared to other time points. These results of neutrophil chemokines may reflect the normal early expression in these two particular wound sites, as demonstrated previously,5 but it is not the same in the case of skin and small bowel wounds. These results may support the faster healing process in colonic wounds in comparison with skin wounds, as demonstrated previously,21 ; 22 which has been attributed to the differences in the time course, and degree of cytokine and growth factor expressions.26 Similarly, Loram et al27 have shown that concentrations of proinflammatory cytokines (IL-1β, TNF-α, IL-6, and CINC-1) remained elevated for 4–8 days after surgery of skin in rats. They concluded that the expression of these cytokines is not essential for the initial development of postoperative primary hyperalgesia.
Neutrophils are dominant in the wound site during the first days after surgery.4 By contrast, macrophages (activated monocytes) reached their maximum levels on Day 2. MCP-1 is a member of β-chemokines, which plays a pivotal role in the recruitment of effector monocytes into the inflamed tissues,23 MCP-1 can be produced by a variety of cell types, including endothelial cells, epithelial cells, smooth muscle cells, fibroblasts, and monocytes.28 Mazzucchelli et al29 found an increase in the number of MCP-1-expressing cells in the inflamed intestinal mucosa of patients with inflammatory bowel diseases. Increased MCP-1 mRNA and protein content in inflamed colonic mucosa were also reported.30 ; 31 Another study showed that mucosal smooth muscle and endothelial cells in the inflamed intestine of inflammatory bowel disease patients express MCP-1.32 Other researchers have observed that MCP-1 production was significantly lower in the inflamed intestinal smooth muscle cells than in normal cells.33 Gauglitz et al16 demonstrated that plasma MCP-1 levels were elevated at 24–48 hours and started to decrease 6 days postburn injury. In our present study, expression profiles of MCP-1 in the four wound sites were not similar. On the one hand, MCP-1 was observed to be elevated on Day 1 and Day 3 in skin and muscle tissues and then declined throughout the length of the study. On the other hand, it showed a considerable lag prior to displaying a significant increase on Day 5 for colonic wounds. A similar result was observed for this chemokine in small bowel wounds, where it increased on Day 5 and remained elevated throughout the length of the study. In this study, MCP-1 levels in small bowel and colon were observed to follow a kinetic profile similar to that of human skin.5
TGF-β is released by several types of cells such as neutrophils, macrophages, fibroblasts, and keratinocytes.10 TGF-β is involved in all phases of wound healing, where it inhibits the inflammatory process and induces fibroblast proliferation to form the granulation tissue, deposition of extra cellular matrix proteins, and angiogenesis.34 ; 35 Although in a previous study, the authors failed to show an increase in TGF-β levels for certain reasons,13 our study showed a significant increase in TGF-β levels in the small bowel wounds on Day 5, which may be consistent with the findings of Buckmire et al.36 This will interpret the early healing events in intestinal wounds in comparison to skin wounds.21 ; 26
In conclusion, the present study has shown an early expression of CINC-1 and a late expression of MCP-1 in colonic wounds, but no significant increase in TGF-β levels was observed. Further studies should seek additional information on the kinetics of changes in chemokine and cytokine levels postsurgery, and the association (or lack thereof) of changes in chemokine and cytokine levels with some other physiological parameters such as prostaglandins, oxidant stress, and antioxidant enzymes. We hope that this present study provides a very good starting point for more in-detail observations by other investigators, to improve the understanding of the biology of wound healing as a guide for the development and use of cytokine/anticytokine and growth factor therapies in the preoperative period.
Acknowledgments
This work was supported by King Abdulaziz City for Science and Technology (KACST) grant number: ARP-28-82. We would like to thank Mr. Sabirin S. for his help in running the samples.
References
- 1 D.A. Dubay, M.G. Franz; Acute wound healing: the biology of acute wound failure; Surg Clin North Am, 83 (2003), pp. 463–481
- 2 A. Pronio, A. Di Filippo, P. Narilli, et al.; [Anastomotic dehiscence in colorectal surgery. Analysis of 1290 patients]; Chir Ital, 59 (2007), pp. 599–609 [Article in Italian]
- 3 B.C. Nwomeh, D.R. Yager, I.K. Cohen; Physiology of the chronic wound; Clin Plast Surg, 25 (1998), pp. 341–356
- 4 R.A. Clark, P.M. Henson; Overview and General Considerations of Wound Repair. The Molecular and Cellular Biology of Wound Repair; Plenum Press, New York (1988)
- 5 E. Engelhardt, A. Toksoy, M. Goebeler, et al.; Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing; Am J Pathol, 153 (1998), pp. 1849–1860
- 6 R. Gillitzer, M. Goebeler; Chemokines in cutaneous wound healing; J Leukoc Biol, 69 (2001), pp. 513–521
- 7 D. Cugini, N. Azzollini, E. Gagliardini, et al.; Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion; Kidney Int, 67 (2005), pp. 1753–1761
- 8 H. Nakagawa, A. Ikesue, H. Kato, et al.; Changes in the levels of rat interleukin 8/CINC and gelatinase in the exudate of carrageenin-induced inflammation in rats; J Pharmacobiodyn, 15 (1992), pp. 461–466
- 9 C.D. Ramos, N.E. Heluy-Neto, R.A. Ribeiro, S.H. Ferreira, F.Q. Cunha; Neutrophil migration induced by IL-8-activated mast cells is mediated by CINC-1; Cytokine, 21 (2003), pp. 214–223
- 10 A.J. Singer, R.A. Clark; Cutaneous wound healing; N Engl J Med, 341 (1999), pp. 738–746
- 11 W. Wagner, M. Wehrmann; Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin; J Cell Mol Med, 11 (2007), pp. 1342–1351
- 12 J.A. Attard, M.J. Raval, G.R. Martin, et al.; The effects of systemic hypoxia on colon anastomotic healing: an animal model; Dis Colon Rectum, 48 (2005), pp. 1460–1470
- 13 A. Zubaidi, W.D. Buie, D.A. Hart, D. Sigalet; Temporal expression of cytokines in rat cutaneous, fascial, and intestinal wounds: a comparative study; Dig Dis Sci, 55 (2010), pp. 1581–1588
- 14 F. Takahashi, K.M. Das; Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis; J Clin Invest, 76 (1985), pp. 311–318
- 15 P. Martin; Wound healing—aiming for perfect skin regeneration; Science, 276 (1997), pp. 75–81
- 16 G.G. Gauglitz, J. Song, D.N. Herndon, et al.; Characterization of the inflammatory response during acute and post-acute phases after severe burn; Shock, 30 (2008), pp. 503–507
- 17 M. Hamaguchi, T. Watanabe, K. Higuchi, et al.; Mechanisms and roles of neutrophil infiltration in stress-induced gastric injury in rats; Dig Dis Sci, 46 (2001), pp. 2708–2715
- 18 J. Le, J. Vilcek; Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities; Lab Invest, 56 (1987), pp. 234–248
- 19 J.S. Pober, M.A. Gimbrone Jr., L.A. Lapierre, et al.; Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon; J Immunol, 137 (1986), pp. 1893–1896
- 20 J.S. Pober, M.P. Bevilacqua, D.L. Mendrick, et al.; Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells; J Immunol, 136 (1986), pp. 1680–1687
- 21 P. Brasken; Healing of experimental colon anastomosis; Eur J Surg Suppl, 566 (1991), pp. 1–51
- 22 H. Jiborn, J. Ahonen, B. Zederfeldt; Healing of experimental colonic anastomoses. III. Collagen metabolism in the colon after left colon resection; Am J Surg, 139 (1980), pp. 398–405
- 23 A.D. Luster; Chemokines—chemotactic cytokines that mediate inflammation; N Engl J Med, 338 (1998), pp. 436–445
- 24 F. Shibata, K. Konishi, H. Kato, et al.; Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 alpha, CINC-2 beta and CINC-3; Eur J Biochem, 231 (1995), pp. 306–311
- 25 J.J. Oppenheim, C.O. Zachariae, N. Mukaida, K. Matsushima; Properties of the novel proinflammatory supergene "intercrine" cytokine family; Annu Rev Immunol, 9 (1991), pp. 617–648
- 26 M. Schaffer, N. Fuchs, J. Volker, et al.; Differential effect of tacrolimus on dermal and intestinal wound healing; J Invest Surg, 18 (2005), pp. 71–79
- 27 L.C. Loram, A.C. Themistocleous, L.G. Fick, P.R. Kamerman; The time course of inflammatory cytokine secretion in a rat model of postoperative pain does not coincide with the onset of mechanical hyperalgesia; Can J Physiol Pharmacol, 85 (2007), pp. 613–620
- 28 A. Yadav, V. Saini, S. Arora; MCP-1: chemoattractant with a role beyond immunity: a review; Clin Chim Acta, 411 (2010), pp. 1570–1579
- 29 L. Mazzucchelli, P. Loetscher, A. Kappeler, et al.; Monocyte chemoattractant protein-1 gene expression in prostatic hyperplasia and prostate adenocarcinoma; Am J Pathol, 149 (1996), pp. 501–509
- 30 G. McCormack, D. Moriarty, D.P. O'Donoghue, et al.; Tissue cytokine and chemokine expression in inflammatory bowel disease; Inflamm Res, 50 (2001), pp. 491–495
- 31 H.C. Reinecker, E.Y. Loh, D.J. Ringler, et al.; Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa; Gastroenterology, 108 (1995), pp. 40–50
- 32 M.C. Grimm, S.K. Elsbury, P. Pavli, W.F. Doe; Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa; J Leukoc Biol, 59 (1996), pp. 804–812
- 33 M.A. Alzoghaibi, S.W. Walsh, A. Willey, A.A. Fowler III, M.F. Graham; Linoleic acid, but not oleic acid, upregulates the production of interleukin-8 by human intestinal smooth muscle cells isolated from patients with Crohns disease; Clin Nutr, 22 (2003), pp. 529–535
- 34 C. Margadant, A. Sonnenberg; Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing; EMBO Rep, 11 (2010), pp. 97–105
- 35 B.J. Faler, R.A. Macsata, D. Plummer, L. Mishra, A.N. Sidawy; Transforming growth factor-beta and wound healing; Perspect Vasc Surg Endovasc Ther, 18 (2006), pp. 55–62
- 36 M.A. Buckmire, G. Parquet, S. Greenway, R.H. Rolandelli; Temporal expression of TGF-beta1, EGF, and PDGF-BB in a model of colonic wound healing; J Surg Res, 80 (1998), pp. 52–57
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?