- This retrospective study was performed at the University of Georgia College of Veterinary Medicine, Veterinary Teaching Hospital.
Abstract
The use of mycophenolate mofetil (MMF) for a variety of immune-mediated diseases in veterinary medicine has been described. However, there is only a small number of cases documenting its use in dogs with meningoencephalomyelitis of unknown aetiology (MUE). We hypothesized that the use of MMF and corticosteroids in dogs with MUE results in comparable survival data to other published treatment protocols and is associated with limited adverse effects. A retrospective study of medical case records of dogs clinically diagnosed with MUE recorded signalment, neuroanatomic localization, magnetic resonance imaging findings, cerebrospinal fluid analysis results, medications administered, follow-up neurologic examinations, survival and adverse events. Variables were compared between dogs which were treated with MMF within 30 days of diagnosis (immediate group) vs. dogs in which MMF therapy was started >30 days after diagnosis (delayed group). Twenty-five cases of MUE were identified. The overall median survival time from diagnosis was 731 days (range 43–1672 days). After 1 month of MMF treatment, 92% of dogs showed improvement on a neurological examination. There was no significant effect of any recorded parameter on survival, including delayed vs. immediate initiation of MMF treatment. Dogs with delayed treatment had significantly lower clinical remission rates than dogs with immediate treatment at 6 months after starting MMF. Adverse events were identified in two cases (8%) and were characterized by mild gastrointestinal signs (vomiting and decreased appetite). Administration of MMF appears safe in dogs with MUE. The use of MMF results in comparable survival times to alternate immunosuppressive protocols.
Introduction
Canine meningoencephalomyelitis of unknown aetiology (MUE) is a broad term which describes a multitude of inflammatory central nervous system (CNS) diseases. Magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) analysis results are compatible with inflammatory CNS disease. A histopathologic diagnosis is generally not available because of the difficulty in obtaining material suitable for histopathology pre-mortem (Zarfoss et al. 2006; Smith et al. 2009; ). At least, one-third of inflammatory CNS disease is classified as MUE, which can include specific diseases such as granulomatous meningoencephalitis (GME), necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE) and idiopathic tremor syndrome (Tipold 1995; Tipold et al. 1995; Smith et al. 2009). As part of the diagnostic investigation, infectious aetiologies are usually excluded with serum and CSF titres and polymerase chain reaction (PCR) testing, although infectious aetiologies are infrequent causes of inflammatory CNS disease in the USA and Europe (Schatzberg et al. 2005; Barber et al. 2012). In the absence of an infectious or neoplastic aetiology, an immune-mediated process is suspected in cases of MUE. Similar to other immune-mediated diseases, canine MUE presents a considerable therapeutic challenge, in part due to the fact that the primary inciting cause of the inflammation is usually unconfirmed.
Many immunosuppressive and cytotoxic protocols have been investigated for the treatment of MUE (Zarfoss et al. 2006; Adamo et al. 2007; Jung et al. 2007, 2013; Menaut et al. 2008; Pakozdy et al. 2009; Smith et al. 2009). Glucocorticoids, cytosine arabinoside, azathioprine, lomustine, procarbazine, leflunomide and cyclosporine have all been used to treat MUE (Gregory et al. 1998; Munana & Luttgen 1998; Zarfoss et al. 2006; Adamo et al. 2007; Coates et al. 2007; Menaut et al. 2008; Pakozdy et al. 2009; Flegel et al. 2011). Mycophenolate mofetil (MMF) has been documented for use in other immune-mediated diseases, and has been proposed for use in inflammatory CNS disease (Lujan Feliu-Pascual et al. 2008; Whitley & Day 2011).
The prognosis for dogs with MUE was considered poor (Zarfoss et al. 2006). Reported median survival times with steroid treatment alone range from 36 to 602 days (Jung et al. 2007; Granger et al. 2010; Flegel et al. 2011; Cornelis et al. 2013; Mercier & Barnes Heller 2015). When an additional immunosuppressive or cytotoxic therapy protocol is combined with corticosteroids, median survival is reported to be 240–1834 days (Gregory et al. 1998; Zarfoss et al. 2006; Adamo et al. 2007; Coates et al. 2007; Menaut et al. 2008; Pakozdy et al. 2009; Smith et al. 2009; Wong et al. 2010; Jung et al. 2013). Therefore, an added immunosuppressive may convey a benefit in the treatment of MUE in dogs (Granger et al. 2010). However, there are no prospective controlled studies comparing the efficacy of one secondary immunosuppressive to another (Granger et al. 2010).
Mycophenolate mofetil (CellCept®; Genentech USA Inc. San Francisco, CA, USA) is the pro-drug of mycophenolic acid, an inhibitor of the enzyme inosine 5′-monophosphate dehydrogenase (IMPDH). Lymphocytes require IMPDH in the de novo synthesis of guanosine monophosphate for purines (Braun et al. 2002; Dewey et al. 2010). Mycophenolic acid inhibits T- and B- lymphocyte production, suppressing both cell-mediated and humoral immune responses (Storb et al. 1997; Yu et al. 1998; Barten et al. 2002, 2002 Sep; Braun et al. 2002; Howard et al. 2002; Lupu et al. 2006; Bacek & Macintire 2011).
In cats and dogs, MMF has been used to prevent rejection associated with organ transplantation, and for systemic immune-mediated diseases (Creevy et al. 2003; Broaddus et al. 2006; Yuki et al. 2007; Abelson et al. 2009; Dewey et al. 2010; Bacek & Macintire 2011; Wang et al. 2013). Adverse events reported in dogs and cats include weight loss, diarrhoea, papillomatosis and allergic reactions (Hood & Zarembski 1997; Yu et al. 1998; Lupu et al. 2006; Bacek & Macintire 2011; Wang et al. 2013). Anticipated advantages of MMF over other immunosuppressive medications include the availability of oral and parenteral forms, rapid onset of action, and reduced myelosuppression or hepatotoxicity (Langman et al. 1996; Yu et al. 1998; Whitley & Day 2011).
The aim of this retrospective study was to describe the use of MMF in client-owned dogs with MUE. We hypothesized that (i) there would be comparable survival times between dogs treated with MMF vs. other published immunosuppressive protocols, and (ii) adverse events would be infrequent in MMF-treated dogs. We also aimed to identify any risk factors that might be associated with clinical response to MMF therapy.
Materials and methods
Medical records (2007–2012) were searched to identify dogs diagnosed with MUE and treated with MMF. Inclusion criteria were: (i) multifocal or focal neuroanatomical localization, (ii) CSF with predominantly mononuclear or lymphocytic inflammation (defined as a pleocytosis comprising at least 50% mononuclear cells with no other leukocyte exceeding 25%), (iii) MRI of the CNS consistent with focal or multifocal disease most compatible with a non-infectious, inflammatory aetiology, and (iv) at least 1 month of follow-up information after initiating MMF therapy; however, dogs which died within 1 month were included in the survival analysis. Dogs surviving <1 month were excluded from other statistical analyses as these relied upon follow-up neurological examinations starting 1 month post-treatment. To exclude aseptic suppurative meningitis, dogs were removed from the study if the CSF revealed a neutrophilic pleocytosis (defined as a pleocytosis comprising at least 25% neutrophils).
All dogs were anaesthetized and imaged using a 3.0 T Signa GE HDx MRI unit (GE Healthcare, Waukesha, WI, USA), with a single channel extremity coil. Dogs were positioned in sternal recumbency. MR images of brain and cervical spinal cord from C1 through C7 were obtained in the sagittal and transverse planes using the following pulse sequences: T1-weighted fluid attenuated inversion recovery (T1W FLAIR), T2-weighted (T2W) and T2W FLAIR. Following the intravenous administration of 0.1 mmol kg−1 of gadopentetate, dimeglumine (Magnevist® injection; Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ, USA), further T1W FLAIR sequences were obtained. The following MRI parameters were used: field of view of 721 × 530, slice thickness of 2 mm on sagittal images, slice thickness of 3 mm on transverse images with an interslice gap of 0 mm and matrix size of 512 × 512. Cerebrospinal fluid was obtained from the cisterna magna after the MRI, while the dog was under the same anaesthesia. Cytologic analysis of the CSF was performed by a board-certified clinical pathologist on the same day. Infectious disease testing for Toxoplasma gondii, Neospora caninum, Cryptococcus neoformans, canine distemper virus, Ehrlichia canis and Rickettsia rickettsii were performed by PCR or serologic assays on CSF or blood in some of the cases and the results of these tests were recorded when performed.
Data collected for each dog included signalment, presenting complaint(s), body weight and body condition score; presenting neuroanatomical localization; severity of neurologic clinical signs (classified according to the following scale: 1 = pain only, 2 = ambulatory paresis, 3 = non-ambulatory paresis, 4 = plegia, and 5 = plegia with absent nociception); and results of any infectious disease testing; CSF analysis including cell count, protein content and cytological evaluation; diagnostic imaging including MRI findings, duration of hospitalization, and survival from the initiation of MMF therapy.
Data specific to each dogs MMF therapy were also collected. These data included the date that MMF therapy was initiated, to allow separation of the dogs into two groups. Those which received MMF within 30 days of being diagnosed with MUE were defined as the ‘immediate’ therapy group; those which received MMF >30 days following their MUE diagnosis were defined as the ‘delayed’ therapy group. Additionally, immunosuppressive medications other than MMF used in each patient were recorded. All patients received corticosteroids as the primary immunosuppressive medication. Whether MMF was the first, second, or in some cases, third or fourth adjunctive immunosuppressive agent added to the regimen was recorded. When available, the reason why additional immunosuppressive medications were utilized was recorded. If the record stated a new medication was being used due to a worsening or unchanged neurologic examination, this was recorded as the patient having failed the previously medication regimen. Data collected also included the dose of MMF used, and any adverse events noted. Adverse events potentially attributable to MMF or other immunosuppressive therapy were extracted from ICU flow sheets, communication logs and/or clinicopathologic data. Referring veterinarians and clients were contacted by phone for details of patient outcome and adverse events. All phone calls were performed by the primary author, and all occurred 6 months or more after all cases were diagnosed.
Data from follow-up neurologic examinations, when available, were recorded at 1, 2 and 6-months following the initiation of MMF therapy. At each time point, the neurologic examination findings were classified based on how they compared to the previous examination. All follow-up neurologic examinations were performed at the authors’ institution (Veterinary Teaching Hospital, University of Georgia) by either a faculty or house officer of the neurology service. For this study, follow-up examinations were classified as worse, unchanged, improved or normal (implying a clinical remission).
Chi-square analysis was performed to test for associations between various risk factors and neurologic examination classification as described above at 1, 2 and 6 months after starting MMF for all dogs and for dogs with delayed and immediate treatment separately. If the sample size was <24, then Fishers exact test was used. Students t-tests were used to compare risk factors for dogs with delayed and immediate treatment separately. The folded form F statistic was used to test if variances were equal between groups. If unequal, then Satterwaithes approximation for degrees of freedom for the students t-test was used.
Delayed treatment was considered along with each of the other risk factors in a multiple logistic regression to assess impact on the odds of clinical remission at 6 months after starting MMF. These risk factors included age at the onset of clinical signs, duration of clinical signs, neuroanatomic localization, presence of neck pain or cranial nerve deficits, severity of clinical signs and the use of additional immunosuppressive medications.
Kaplan–Meier survival curves for survival time were constructed to calculate median survival for all dogs. Dogs still living at the time of statistical analysis were censored from the formulation of the Kaplan–Meier curve. A log-rank test was used to test if there was a difference in survival probability between dogs for various risk factors. Cox proportional hazards regression was used to test for relationships of various risk factors to survival probability.
All tests were two sided and the significance level was P = 0.05. All statistical analyses were performed using commercially available software (SAS v9.2; SAS Institute Inc. Cary, NC, USA).
Results
Analysis of medical records yielded 30 cases meeting the initial search criteria. Upon review, three cases were excluded due to lack of CSF collection (n = 2) and loss to follow-up within 1 month of initiating MMF therapy (n = 1). Two dogs were killed at 1 and 6 days after starting mycophenolate, respectively. Reason for killing cited in the medical record included financial and prognostic concerns, as the neurologic status of both dogs was not improving on therapy. Neither dog was noted to develop worsening clinical signs. Both of these dogs were included in the survival analysis. Twenty-five dogs fulfilled the inclusion criteria. Affected breeds included the Maltese (n = 5), Chihuahua, Dachshund, and Yorkshire terrier (n = 3), Pomeranian, Jack Russell terrier, and Miniature Poodle (n = 2), Boston terrier, Saluki, and Italian greyhound (n = 1), and two mixed breed dogs. Fourteen dogs were female, one of these being intact. Eleven dogs were male, four of these being intact. Age at presentation varied from 4 months to 9 years (median age: 4.1 years). The median body weight was 3.8 kg (range 1.6–27 kg), with a median body condition score of 5/9 (range 3–7). Diagnostics for infectious diseases were pursued in 6/25 cases (24%) and found to be negative in all cases.
Of the presenting neurologic signs, paraparesis (n = 8), ataxia (n = 7) and seizures (n = 5) were the most common. Other presenting neurologic signs included head tilt, circling, visual deficits, neck pain and behavioural changes. The most common neuroanatomic localization was the prosencephalon (n = 12). Additional common neuroanatomic sites affected included the vestibular system (n = 6), the first cervical spinal segment (C1–C5; n = 6) and the thoracolumbar spinal segment (T3–L3; n = 5). Other neuroanatomic sites affected included the cerebellum (n = 3), brainstem (n = 3), and the cervicothoracic (C6–T2; n = 1) and lumbosacral (L4–S2; n = 1) spinal segments. Nine of the cases (36%) had a multifocal neuroanatomic localization. The duration of clinical signs prior to diagnosis ranged from 1 day to 2 years (median 4 weeks).
The dogs received a mean dose of 20.05 mg kg−1day−1 (range 18.40–21.15 mg kg−1 day−1) of MMF divided twice daily, given orally and/or parenterally. When given intravenously, the dosage was administered over 20 min using a syringe pump (MedFusion® 3500 Syringe Pump; Smiths Medical, St. Paul, MN, USA). Additional immunosuppressives used in these cases included prednisone (n = 25), cytosine arabinoside (n = 13), cyclosporine (n = 12), leflunomide (n = 1) and lomustine (n = 1). In all 25 cases, prednisone was the first immunosuppressive medication used, and all initial doses were administered at 2 mg kg−1 day−1 or higher. Changes in prednisone dosage over time were not included. In 11 cases, MMF was the first adjunctive immunosuppressive medication used. Additional immunosuppressive medication information is summarized in Table 1.
| Dog | Breed | Gender | Adjunctive medication #1* | Adjunctive medication #2* | Adjunctive medication #3* | Additional adjunctive medications* |
|---|---|---|---|---|---|---|
| 1 | Dachshund | MN | MMF | – | – | – |
| 2 | Dachshund | M | MMF | – | – | – |
| 3 | Maltese | F | MMF | – | – | – |
| 4 | Saluki | M | MMF | – | – | – |
| 5 | Italian Greyhound | MN | MMF | – | – | – |
| 6 | Dachshund | FS | MMF | – | – | – |
| 7 | Jack Russell Terrier | FS | MMF | – | – | – |
| 8 | Chihuahua | MN | MMF | – | – | – |
| 9 | Mixed Breed Dog | FS | MMF | – | – | – |
| 10 | Yorkshire Terrier | MN | MMF | CSA | – | – |
| 11 | Pomeranian | FS | MMF | CSA | – | – |
| 12 | Maltese | FS | CSA | MMF | – | – |
| 13 | Jack Russell Terrier | FS | CSA | MMF | – | – |
| 14 | Chihuahua | M | Cyto | MMF | – | – |
| 15 | Miniature Poodle | FS | Cyto | MMF | – | – |
| 16 | Maltese | FS | Cyto | MMF | – | – |
| 17 | Mixed Breed Dog | FS | Cyto | MMF | – | – |
| 18 | Pomeranian | MN | Cyto | MMF | CSA | – |
| 19 | Maltese | FS | Cyto | MMF | CSA | Lomustine, Leflunomide |
| 20 | Yorkshire Terrier | MN | Cyto | MMF | CSA | – |
| 21 | Chihuahua | FS | Cyto | MMF | CSA | – |
| 22 | Maltese | FS | Cyto | CSA | MMF | – |
| 23 | Miniature Poodle | M | Cyto | CSA | MMF | – |
| 24 | Boston Terrier | FS | Cyto | CSA | MMF | – |
| 25 | Yorkshire Terrier | MN | Cyto | CSA | MMF | – |
MMF, mycophenolate mofetil; CSA, cyclosporine A; Cyto, cytosine arabinoside. *All 25 dogs were treated with corticosteroids as their primary therapy, with the medications depicted in the table used as adjunctive agents. | ||||||
Adverse events were reported in two cases (8%). In one case, the client reported several episodes of vomiting within the first week of starting MMF. In the other case, the client reported an occasional decrease in appetite noted after starting MMF. Each instance of decreased appetite was self-limiting within about 24–48 h, and never required additional medications or hospitalization. MMF was not discontinued due to adverse events in either case.
In eleven cases (44%), MMF therapy was considered immediate in that it was initiated within 30 days of diagnosis of MUE (median 2 days; range 0–26 days). In the other fourteen cases, MMF therapy was considered delayed in that it was initiated >30 days following the diagnosis of MUE (median 215.5 days; range 34–966 days). Follow-up neurologic examinations are summarized in Table 2. All 25 dogs were assessed at 1 month following the initiation of MMF therapy. At this time, one dog was in a clinical remission. Twenty-four dogs (96%) were assessed at 2 months following initiation of MMF; the remaining dog had been killed due to worsening neurologic disease. Follow-up information was available for 18 dogs (72%) at 6 months following the initiation of MMF; the remaining six dogs had been killed due to worsening neurologic disease. Of the six killed dogs, three were in the immediate treatment group, while the other three were in the delayed treatment group.
| Neurologic examination classification | |||||
|---|---|---|---|---|---|
| Clinical remission | Improved | Unchanged | Worse | Total | |
| 1 month follow-up | 1 | 21 | 2 | 1 | 25 |
| 2 month follow-up | 1 | 14 | 7 | 2 | 24 |
| 6 month follow-up | 3 | 2 | 12 | 1 | 18 |
Dogs with delayed treatment had significantly lower clinical remission rates (0/11 = 0%) than dogs with immediate treatment (3/7 = 43%) at 6 months after starting MMF (P = 0.043). When delayed treatment was considered along with other risk factors in a multivariate analysis, none of these factors showed a significant effect on the chances of clinical remission at 6 months after starting MMF.
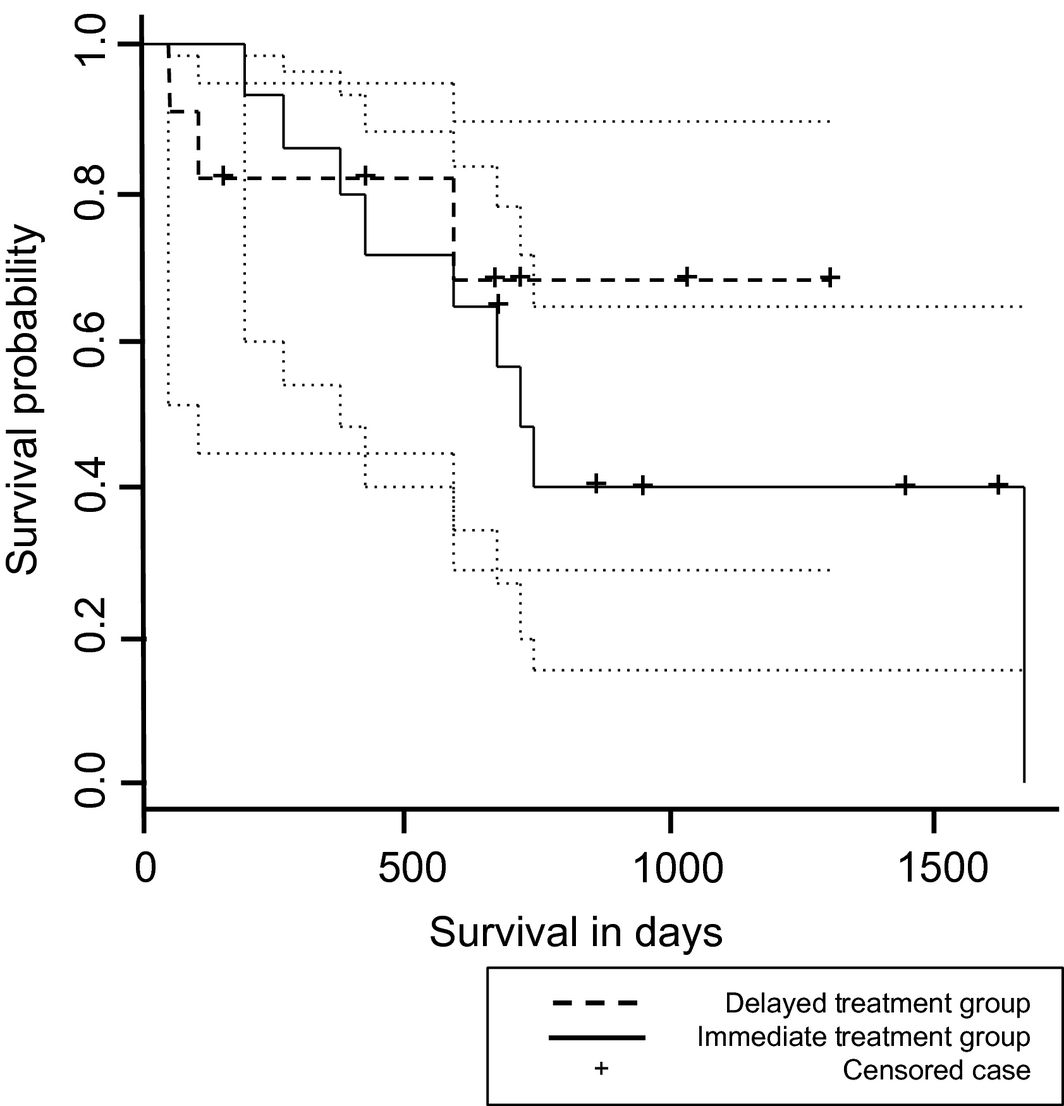
The median survival time from the time of diagnosis was 731 days [95% confidence interval (CI); range 582–1672 days] in all dogs. Within the treatment groups, the median survival time from diagnosis was 585.5 days (95% CI; minimum 2 days, maximum not estimable) in the immediate treatment group and 713 days (95% CI; range 187–1672 days) in the delayed treatment group. The median survival time from the time of MMF initiation was 620 days (95% CI; minimum 2 days, maximum not estimable) in all dogs. The median survival time from MMF initiation was 584 days (95% CI; minimum 2 days, maximum not estimable) in the immediate treatment group and 284 days (95% CI; minimum 63 days, maximum not estimable) in the delayed treatment group. There was no significant difference in the survival data between treatment groups. Kaplan–Meier survival curves are depicted in Fig. 1.
|
|
|
Figure 1. Kaplan–Meier survival curve for dogs treated with mycophenolate mofetil in the delayed treatment and immediate treatment groups. No difference in median survival time was detected. Dotted lines represent the 95% confidence intervals for each treatment group. Vertical ticks represent time of censoring of dogs lost to follow-up. |
Discussion
The results of this study fulfilled the hypothesis that the survival times in dogs with MUE treated with corticosteroids and MMF are comparable to those documented with other published immunosuppressive protocols. This retrospective study showed that the median survival time after starting MMF was 620 days. The inclusion criteria required that all dogs survived a minimum of 30 days after starting MMF, but in the study, 96% (24/25) of the included dogs survived >2 months after the initiation of MMF. The included cases were stratified based on whether or not MMF was started within 30 days of the diagnosis of MUE (immediate group) or after this time point (delayed group). Our study did not find any correlation between survival and the immediate vs. delayed initiation of MMF therapy. The immediacy with which MMF was started was not shown to have a statistically significant impact on overall survival time; however, the dogs which received MMF within 30 days of diagnosis did demonstrate a higher likelihood of clinical remission upon neurologic examination at the 6 month follow-up when compared to the dogs receiving delayed MMF therapy. The lack of correlation could be due to the fact that all cases received immunosuppressive doses of glucocorticoids prior to starting MMF, and in 56% of cases (14/25), additional adjunct immunosuppressive medications were attempted and noted to fail prior to the initiation of MMF. The survival benefit from these additional immunosuppressive agents was not investigated in this study. Interestingly, we did not find any correlation between the order in which MMF was started as part of the immunosuppressive protocol and survival.
Our study resulted in survival data that are most comparable to the studies investigating the use of prednisone in combination with cyclosporine (MST 620 days) or cytosine arabinoside (MST 531 days) (Zarfoss et al. 2006; Pakozdy et al. 2009). Similar to our study, these studies are limited by their retrospective nature and by the fact that the immunosuppressive medication being investigated is used as a part of multi-drug therapy. Nearly, half of our study population (12 dogs) were still alive as of the date of this publication; therefore, the median survival time could be longer or shorter than reported.
The literature includes many studies investigating the use of adjunctive immunosuppressive medications in addition to corticosteroids, but the benefit of their use is not clear. While median survival time is commonly used to demonstrate the superiority of adjunctive immunosuppressive agents, it would appear that the initial response to therapy may be an important predictor of the pharmacological needs of these patients. In one study, it was shown that when MUE was not immediately fatal, the disease followed a corticosteroid-responsive and potentially self-limiting course, without the need for adjunctive immunosuppressive agents (Cornelis et al. 2013).
To the authors’ knowledge, this is the first study to look at the impact of lag time between diagnosis and initiation of the medication to be studied on overall treatment efficacy and survival. If an association between immediate MMF therapy and outcome were to be upheld, more than one explanation for that effect would be possible. It could be that MMF is superior therapy for MUE, and prompt initiation of therapy improves long-term outcome. Or it could be that those dogs likely to improve fully also begin to improve quickly, giving the clinician no indication for addition of third or fourth immunosuppressive agents. That is to say, it is possible that it is not that delayed use of MMF is associated with less successful outcome, but that a requirement for a third or fourth agent is typical of those dogs with less successful outcomes.
Drug-related adverse events are reported rarely in the treatment of MUE. Due to the fact that a clear survival benefit was not demonstrated in our population of dogs treated with MMF, it was important to evaluate reported adverse events as this could impact clinician decision-making when selecting an adjunctive immunosuppressive agent. Adverse events associated with the use of MMF were infrequent in this population of dogs. In our review of the medical records and follow-up interviews with referring veterinarians and clients, only two dogs were identified to have adverse events that could have been associated with their MMF therapy. These adverse events were primarily gastrointestinal in origin, with one being vomiting and the other inappetence, and were self-limiting in both cases. While a direct association could not be made, these findings are consistent with other studies on the use of MMF in veterinary patients. (Lange et al. 2008; Dewey et al. 2010; Wang et al. 2013). Gastrointestinal upset is also the most common side effect noted with the use of MMF in people, but additional adverse effects reported include teratogenicity, increased risk of lymphoma, allergic reactions and headaches (Braun et al. 2002; Bacek & Macintire 2011; Hoeltzenbein et al. 2012). While we are reporting gastrointestinal adverse events only, the 6 month follow-up period would not be long enough to evaluate for the development of lymphoma. Also, it is possible that some adverse events were not recalled by the owner or written in medical records. Clinicopathologic data was not reliably available in these patients during the follow-up period, and therefore it is possible that adverse events manifesting as changes in biochemical parameters were not identified.
There were two dogs that were killed within 1 week of starting mycophenolate therapy; however, new or worsening clinical signs were not documented after this medication was initiated. The neurologic examination of these dogs did not improve in response to mycophenolate, so had killing not been elected, they may have benefitted from a different immune-suppressive medication or further diagnostic evaluation. It is unclear if mycophenolate could have contributed to the choice of killing in these patients, but no adverse effects were reported which could be temporally referable to the initiation of MMF.
Advantages of MMF include its oral and parenteral formulations, bioavailability and rapid onset of action, as well as its relative affordability. The availability of a parenteral formulation would be particularly helpful in those patients who are severely neurologically affected in the initial stages of their therapy and may not be eating reliably, making oral administration of medications difficult. The availability of an oral formulation with similar bioavailability allows for a reliable transition from injectable medication to oral administration of MMF when the patient is discharged from the hospital.
There are several limitations to this study. None of the cases included in this study had a histopathologic diagnosis prior to the initiation of therapy. While diagnostic efforts were made to exclude all other disease possibilities, a definitive diagnosis was not made ante-mortem in any of the cases. Without a definitive diagnosis, it is possible that cases of GME, NLE and NME were included and all termed MUE. Previous studies have excluded NLE and NME from investigation due to the difference in inflammatory pattern and histologic lesions and distribution (Granger et al. 2010). As samples for histology were infrequently obtained in our study, a diagnosis of NME or NLE was not an exclusion criterion. This may mean that MMF has different efficacy depending on the type of inflammatory CNS disease, but this is beyond the scope of our study.
Another limitation is that infectious disease testing was not performed in all cases, so this category of disease could not be excluded in all cases. Current infectious disease diagnostic practices lack sensitivity for detection of CNS disease, but is still an important category of disease to investigate as a cause for meningoencephalomyelitis (Schatzberg et al. 2003; Barber et al. 2010). The severity of neurologic signs often warrants prioritization of cross-sectional imaging and collection of CSF rather than waiting on the results infectious disease testing. If concern for infectious differentials is increased based on MRI or CSF findings, this is often when targeted infectious disease diagnostics are pursued.
Lack of disease confirmation is a common limitation in the studies investigating MUE, as a histopathologic diagnosis is not usually obtained. Of the studies investigating the use of other adjunctive immunosuppressive agents and their use in MUE, only three have included patients with a histopathologic diagnosis (Munana & Luttgen 1998; Adamo et al. 2007; Flegel et al. 2011). The case selection criteria in this study required characteristic MRI findings and CSF abnormalities of MUE in order to narrow the chance of diagnostic error. Post-mortem examinations were performed in six dogs. All of these post-mortem examinations identified macrophagic and/or lymphoplasmacytic inflammation within the CNS.
Due to the retrospective analysis, the heterogeneity of immunosuppressive therapy within the population prevents investigation of MMF as a sole adjunctive immunosuppressive agent. While studies exist that investigate the use of an adjunctive immunosuppressive agent compared to corticosteroids alone, there is a dearth of published information that directly compares different adjunctive therapies (Schatzberg et al. 2005; Zarfoss et al. 2006; Adamo et al. 2007; Coates et al. 2007; Menaut et al. 2008; Wong et al. 2010).
Several challenges to creation of a prospective study exist. Withholding corticosteroid therapy from MUE patients would be unethical; therefore, a double-blinded, placebo-controlled study of MMF vs. no therapy should not be performed. There is not an accepted gold standard two-drug immunosuppressive protocol for MUE, therefore it is unclear which two-drug combination should be used as the comparison against corticosteroids plus MMF. It is also unclear whether or not a two-drug protocol should be the recommendation in cases of MUE. Several recent studies have comparable to improved survival data compared to previous literature using prednisolone as a sole therapy (Flegel et al. 2011; Cornelis et al. 2013; Mercier & Barnes Heller 2015). Therefore, it may now be possible to construct a study comparing the use of MMF to the use of prednisolone alone. A prospective, randomized, double-blinded study comparing the effects of MMF as an adjunctive to corticosteroids, with an alternate adjunctive immunosuppressive for the treatment of MUE is warranted based on our data. This study would allow for direct comparison of survival, adverse events and cost. Ideally, this study would be performed with a larger population than exists in most of the current literature regarding MUE.
Conclusion
In summary, this study describes the use of MMF as an adjunctive immunosuppressive medication in dogs with MUE. The purpose of this retrospective analysis was to investigate historical and clinicopathologic risk factors and their impact on clinical response and survival, and to document adverse effects seen with the use of MMF. Based on our findings, immunosuppression with 10 mg kg−1 MMF, intravenous or orally, every 12 h, appears safe in dogs with MUE. The combination of glucocorticoids and MMF was associated with a comparable clinical response and long-term survival when compared with other adjunctive immunosuppressive therapies in the literature, and may have fewer adverse effects.
Acknowledgement
None.
Source of funding
This study was not funded by any granting or external agency.
Conflicts of interest
The authors disclose no conflict of interest.
Contributions
None.
References
- Abelson A.L., Shelton G.D., Whelan M.F., Cornejo L., Shaw S. & O'Toole T.E. (2009) Use of mycophenolate mofetil as a rescue agent in the treatment of severe generalized myasthenia gravis in three dogs. Journal of Veterinary Emergency and Critical Care19, 369–374.
- Adamo P.F., Rylander H. & Adams W.M. (2007) Ciclosporin use in multi-drug therapy for meningoencephalomyelitis of unknown aetiology in dogs. Journal of Small Animal Practice48, 486–496. PubMed PMID: 17617166.
- Bacek L.M. & Macintire D.K. (2011) Treatment of primary immune-mediated hemolytic anemia with mycophenolate mofetil in two cats. Journal of Veterinary Emergency and Critical Care (San Antonio)21, 45–49. PubMed PMID: 21288293.
- Barber R.M., Li Q., Diniz P.P., Porter B.F., Breitschwerdt E.B., Claiborne M.K.et al. (2010) Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 cases). Journal of Veterinary Internal Medicine24, 372–378. PubMed PMID: 20102497.
- Barber R.M., Porter B.F., Li Q., May M., Claiborne M.K., Allison A.B.et al. (2012) Broadly reactive polymerase chain reaction for pathogen detection in canine granulomatous meningoencephalomyelitis and necrotizing meningoencephalitis. Journal of Veterinary Internal Medicine26, 962–968. PubMed PMID: 22686439.
- Barten M.J., van Gelder T., Gummert J.F., Shorthouse R. & Morris R.E. (2002a) Novel assays of multiple lymphocyte functions in whole blood measure: new mechanisms of action of mycophenolate mofetil in vivo. Transplant Immunology10, 1–14. PubMed PMID: 12182459.
- Barten M.J., van Gelder T., Gummert J.F., Boeke K., Shorthouse R., Billingham M.E.et al. (2002b) Pharmacodynamics of mycophenolate mofetil after heart transplantation: new mechanisms of action and correlations with histologic severity of graft rejection. American Journal of Transplantation2, 719–732. PubMed PMID: 12243493.
- Braun K.P., Glander P., Hambach P., Bohler T., Waiser J., Mai I.et al. (2002) Pharmacokinetics and pharmacodynamics of mycophenolate mofetil under oral and intravenous therapy. Transplantation Proceedings34, 1745–1747. PubMed PMID: 12176560.
- Broaddus K.D., Tillson D.M., Lenz S.D., Niemeyer G.P., Brawner W.R., Welch J.A.et al. (2006) Renal allograft histopathology in dog leukocyte antigen mismatched dogs after renal transplantation. Veterinary Surgery35, 125–135. PubMed PMID: 16472292.
- Coates J.R., Barone G., Dewey C.W., Vitale C.L., Holloway-Azene N.M., Sessions J.K. (2007) Procarbazine as adjunctive therapy for treatment of dogs with presumptive antemortem diagnosis of granulomatous meningoencephalomyelitis: 21 cases (1998–2004). Journal of Veterinary Internal Medicine21, 100–106. PubMed PMID: 17338156.
- Cornelis I, Van Ham L, Kromhout K, Goethals K, Gielen I, Bhatti S. (2013) Sole Prednisolone therapy in canine meningoencephalitis of unknown origin: 45 cases (2006–2012). Proceedings – European College of Veterinary Neurology.
- Creevy K.E., Bauer T.R. Jr, Tuschong L.M., Embree L.J., Silverstone A.M., Bacher J.D.et al. (2003) Mixed chimeric hematopoietic stem cell transplant reverses the disease phenotype in canine leukocyte adhesion deficiency. Veterinary Immunology and Immunopathology95, 113–121. PubMed PMID: 12963272.
- Dewey C.W., Cerda-Gonzalez S., Fletcher D.J., Harb-Hauser M.F., Levine J.M., Badgley B.L.et al. (2010) Mycophenolate mofetil treatment in dogs with serologically diagnosed acquired myasthenia gravis: 27 cases (1999–2008). Journal of the American Veterinary Medical Association236, 664–668. PubMed PMID: 20225980.
- Flegel T., Boettcher I.C., Matiasek K., Oevermann A., Doherr M.G., Oechtering G.et al. (2011) Comparison of oral administration of lomustine and prednisolone or prednisolone alone as treatment for granulomatous meningoencephalomyelitis or necrotizing encephalitis in dogs. Journal of the American Veterinary Medical Association238, 337–345. PubMed PMID: 21281217.
- Granger N., Smith P.M. & Jeffery N.D. (2010) Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. The Veterinary Journal184, 290–297. PubMed PMID: 19410487.
- Gregory C.R., Stewart A., Sturges B., DeManvelle T., Cannon A., Ortega T.et al. (1998) Leflunomide effectively treats naturally occurring immune-mediated and inflammatory diseases of dogs that are unresponsive to conventional therapy. Transplantation Proceedings30, 4143–4148. PubMed PMID: 9865328.
- Hoeltzenbein M., Elefant E., Vial T., Finkel-Pekarsky V., Stephens S., Clementi M.et al. (2012) Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. American Journal of Medical Genetics Part A158A, 588–596. PubMed PMID: 22319001.
- Hood K.A. & Zarembski D.G. (1997) Mycophenolate mofetil: a unique immunosuppressive agent. American Journal of Health-System Pharmacy54, 285–294. PubMed PMID: 9028422.
- Howard J., Hoffbrand A.V., Prentice H.G. & Mehta A. (2002) Mycophenolate mofetil for the treatment of refractory auto-immune haemolytic anaemia and auto-immune thrombocytopenia purpura. British Journal of Haematology117, 712–715. PubMed PMID: 12028047.
- Jung D.I., Kang B.T., Park C., Yoo J.H., Gu S.H., Jeon H.W.et al. (2007) A comparison of combination therapy (cyclosporine plus prednisolone) with sole prednisolone therapy in 7 dogs with necrotizing meningoencephalitis. The Journal of Veterinary Medical Science69, 1303–1306. PubMed PMID: 18176031.
- Jung D.I., Lee H.C., Ha J., Jung H.W., Jeon J.H., Moon J.H.et al. (2013) Unsuccessful cyclosporine plus prednisolone therapy for autoimmune meningoencephalitis in three dogs. Journal of Veterinary Medical Science75, 1661–1665. PubMed PMID: 23955394.
- Lange S., Mueller S.C., Altmann S., Dahlhaus M., Drewelow B., Freund M.et al. (2008) Pharmacokinetics of oral mycophenolate mofetil in combination with CsA in dogs after nonmyeloablative allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation41, 667–674. PubMed PMID: 18084333.
- Langman L.J., Shapiro A.M., Lakey J.R., LeGatt D.F., Kneteman N.M. & Yatscoff R.W. (1996) Pharmacodynamic assessment of mycophenolic acid-induced immunosuppression by measurement of inosine monophosphate dehydrogenase activity in a canine model. Transplantation61, 87–92. PubMed PMID: 8560580.
- Lujan Feliu-Pascual A., Matiasek L., Kneissl S., de Stefani A., Beltran E., De Risio L. (2008) Efficacy of Mycophenolate Mofetil for the treatment of presumptive granulomatous meningoencephalomyelitis: preliminary results. In: Proceeding of the 20th ECVN Congress. J Vet Intern Med. 22(509).
- Lupu M., McCune J.S., Kuhr C.S., Gooley T. & Storb R. (2006) Pharmacokinetics of oral mycophenolate mofetil in dog: bioavailability studies and the impact of antibiotic therapy. Biology of Blood and Marrow Transplantation12, 1352–1354. PubMed PMID: 17162219.
- Menaut P., Landart J., Behr S., Lanore D. & Trumel C. (2008) Treatment of 11 dogs with meningoencephalomyelitis of unknown origin with a combination of prednisolone and cytosine arabinoside. The Veterinary Record162, 241–245. PubMed PMID: 18296666.
- Mercier M., Barnes Heller H.L. (2015) Efficacy of glucocorticoid monotherapy for treatment of canine meningoencephalomyelitis of unknown etiology: a prospective study in 16 dogs. Veterinary Medicine and Science1, 16–22.
- Munana K.R. & Luttgen P.J. (1998) Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982–1996). Journal of the American Veterinary Medical Association212, 1902–1906. PubMed PMID: 9638190.
- Pakozdy A., Leschnik M., Kneissl S., Gumpenberger M., Gruber A., Tichy A.et al. (2009) Improved survival time in dogs with suspected GME treated with ciclosporin. The Veterinary Record164, 89–90. PubMed PMID: 19151407.
- Schatzberg S.J., Haley N.J., Barr S.C., deLahunta A., Olby N., Munana K.et al. (2003) Use of a multiplex polymerase chain reaction assay in the antemortem diagnosis of toxoplasmosis and neosporosis in the central nervous system of cats and dogs. American Journal of Veterinary Research64, 1507–1513. PubMed PMID: 14672429.
- Schatzberg S.J., Haley N.J., Barr S.C., de Lahunta A., Sharp N.J. (2005) Polymerase chain reaction screening for DNA viruses in paraffin-embedded brains from dogs with necrotizing meningoencephalitis, necrotizing leukoencephalitis, and granulomatous meningoencephalitis. Journal of Veterinary Internal Medicine19, 553–559. PubMed PMID: 16095173.
- Smith P.M., Stalin C.E., Shaw D., Granger N., Jeffery N.D. (2009) Comparison of two regimens for the treatment of meningoencephalomyelitis of unknown etiology. Journal of Veterinary Internal Medicine23, 520–526. PubMed PMID: 19645837.
- Storb R., Leisenring W., Anasetti C., Appelbaum F.R., Deeg H.J., Doney K.et al. (1997) Methotrexate and cyclosporine for graft-vs.-host disease prevention: what length of therapy with cyclosporine?Biology of Blood and Marrow Transplantation3, 194–201. PubMed PMID: 9360781.
- Tipold A. (1995) Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: a retrospective study. Journal of Veterinary Internal Medicine9, 304–314. PubMed PMID: 8531175.
- Tipold A., Vandevelde M. & Zurbriggen A. (1995) Neuroimmunological studies in steroid-responsive meningitis-arteritis in dogs. Research in Veterinary Science58, 103–108. PubMed PMID: 7761686.
- Wang A., Smith J.R. & Creevy K.E. (2013) Treatment of canine idiopathic immune-mediated haemolytic anaemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007–2011). Journal of Small Animal Practice54, 399–404. PubMed PMID: 23879827.
- Whitley N.T. & Day M.J. (2011) Immunomodulatory drugs and their application to the management of canine immune-mediated disease. Journal of Small Animal Practice52, 70–85. PubMed PMID: 21265846.
- Wong M.A., Hopkins A.L., Meeks J.C. & Clarke J.D. (2010) Evaluation of treatment with a combination of azathioprine and prednisone in dogs with meningoencephalomyelitis of undetermined etiology: 40 cases (2000–2007). Journal of the American Veterinary Medical Association237, 929–935. PubMed PMID: 20946080.
- Yu C., Seidel K., Nash R.A., Deeg H.J., Sandmaier B.M., Barsoukov A.et al. (1998) Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood91, 2581–2587. PubMed PMID: 9516160.
- Yuki M., Sugimoto N., Otsuka H., Tanahashi S., Katoh M., Hirano T.et al. (2007) Recovery of a dog from aplastic anaemia after treatment with mycophenolate mofetil. Australian Veterinary Journal85, 495–497. PubMed PMID: 23879827.
- Zarfoss M., Schatzberg S., Venator K., Cutter-Schatzberg K., Cuddon P., Pintar J.et al. (2006) Combined cytosine arabinoside and prednisone therapy for meningoencephalitis of unknown aetiology in 10 dogs. Journal of Small Animal Practice47, 588–595. PubMed PMID: 17004951.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?