Summary
Background/Objective
Currently, due to lack of optimal donors, more marginal organs are transplanted. Therefore, there is a high interest to ameliorate preischemic organ preservation, especially for critical donor organs. In this regard, a new histidine-tryptophane ketoglutarate (HTK-N) solution has been designed and its protective efficacy was compared with the standard preservation solutions—University of Wisconsin solution and standard HTK or Custodiol (Bretschneiders solution).
Methods
Seventy-two landrace pigs were included into the study, as donors and recipients. The donor kidneys were perfused during explantation with cold University of Wisconsin solution (n = 12), standard HTK (n = 12), or HTK-N solutions (n = 12), kept in the respective preservation solution at 4°C for 30 hours, implanted in the recipient pigs, and reperfused. The pigs survived in daily control for 7 days. The serum creatinine and blood urea nitrogen were assessed in pre- and postreperfusion phase on the 3rd day and 7th day posttransplantation. Additionally, tissue samples were taken to analyze the histopathological degree of tubular injury and regeneration before and after reperfusion.
Results
The three preservation groups were comparable in age, body weight, and hemodynamic parameters. According to statistical proof, they differed in none of the control parameters.
Conclusion
Although the new preservation HTK solution is in several points a well-thought-out modification of the standard HTK solution, its preservation efficacy, at least for kidney preservation in a pig model for 30 hours, seems to be comparable to the current used solutions. A real advantage, however, could be confirmed in clinical settings, where marginal organs may influence the clinical outcome.
Keywords
ischemia reperfusion injury;kidney transplantation;preservation solution
1. Introduction
Organ preservation plays an important role in solid organ transplantation. It has a great influence on ischemia reperfusion injury and early graft function as well as long-term graft survival.1; 2 ; 3 University of Wisconsin (UW) and histidine-tryptophan ketoglutarate (HTK) solutions are currently used as the routine preservation solutions in many transplant centers world-wide. They have shown about the same efficacy in a remarkable number of studies during the past years.4; 5; 6 ; 7 They have, however, functional limitations in protective efficacy against ischemia reperfusion injury, especially in a warm or even cold ischemic period or in organs with extended donor criteria—the so called marginal organs.8; 9; 10; 11 ; 12
In order to reduce these limitations, a new version of the standard HTK solution, HTK-new solution (HTK-N) has been introduced. This solution differs from the routine HTK with increased antioxidative capacity and cold tolerance by the addition of deferoxamine and LK614. Deferoxamine works intracellularly as an iron chelator, and LK614 is a catalase-mimic and catalyzes the reduction of H2O2 to H2O in cells. In rat heart and liver models the superiority of this new preservation solution could be shown in the preservation of coronary vessel structure, endothelial function, improvement of myocardial contractility and relaxation after heart transplantation, and promising therapeutic strategies for attenuation of cold storage injury.13; 14 ; 15 The underlying hypothesis claims that very small intracellular pools of iron together with H2O2 may play a critical role in the formation of the hazardous free radicals.15; 16; 17; 18; 19 ; 20
Furthermore, there is a certain reduction of histidine buffer in favor of N-acetyl-histidine in HTK-N, assuming that histidine could be degraded to toxic products by reactive oxygen or nitrogen species. Besides, the solution contains very high magnesium and higher calcium concentrations for antagonizing the destabilizing effects of high magnesium in cell membranes by calcium antagonism.21 ; 22 Because mannitol in hepatocytes is not completely impermeable, the osmolyte mannitol is replaced by saccharose.23
In this study, our aim is to evaluate the HTK-N solution in a porcine kidney transplantation (KTx) model with a 30-hour cold ischemia time and to compare it with the standard HTK solution and UW solution.
2. Methods
2.1. Animal rights
The Governmental Committee on Animal Care approved the experiments and animals were given humane care in compliance with institutional guidelines. Following completion of the experimental protocol, the animals were sacrificed with an intravenous injection of kalium chloride (2 mmol/kg) in deep anaesthesia.
2.2. Quality of assurance statement
The study was performed in accordance with the Principles of Good Laboratory Practice, annex of paragraph 19a, section 1 of the German chemical law of July 25, 1994.
2.3. Study design
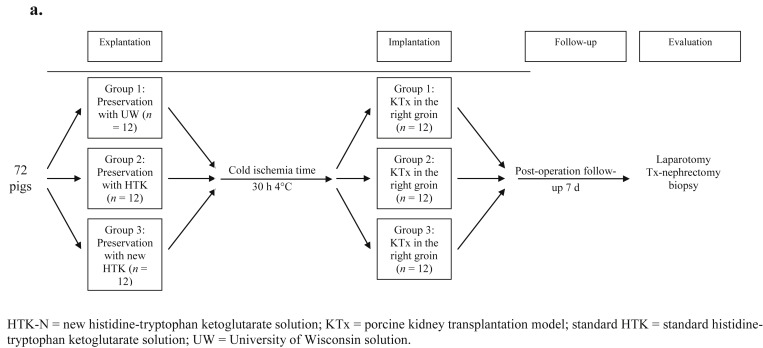
Seventy two Landrace pigs (30–40 kg) were divided into three groups: Group 1 (GI): UW (12 donors and 12 recipients); Group 2 (GII): standard HTK (12 donors and 12 recipients); and Group 3 (GIII): HTK-N (12 donors and 12 recipients). Table 1 summarizes the similarities and differences between these solutions. In each group, the organs were perfused with the respective solution and were implanted after 30 hours of ischemia at 4°C using the standard technique in nephrectomized recipients. The kidney recipients were followed-up for 7 days postoperatively, until final evaluation of blood and tissue parameters (Figure 1 and Table 2).
| Constituents (mmol/L) | UW | Standard HTK | HTK-N |
|---|---|---|---|
| Na+ | 30 | 15 | 16 |
| K+ | 115 | 10 | 10 |
| Mg++ | 5 | 4 | 8 |
| Ca++ | 0 | 0.01 | 0.02 |
| Cl− | — | 50 | 30 |
| SO42− | 5 | — | — |
| Lactobionate | 100 | — | — |
| HPO4 | 20 | — | — |
| H2PO4 | 5 | — | — |
| Histidine | — | 180 | 124 |
| Histidine·HCl | — | 18 | — |
| N-acetylhistidine | — | — | 57 |
| Aspartate | — | — | 5 |
| Tryptophan | — | 2 | 2 |
| Oxoglutarate | 1 | 1 | 2 |
| L-arginine | — | — | 3 |
| Glycine | — | — | 10 |
| L-alanine | — | — | 5 |
| Saccharose | — | — | 33 |
| Raffinose | 30 | — | — |
| Manitol | — | 30 | — |
| Glutatione | 3 | — | — |
| Adenosine | 5 | — | — |
| HAES | 50 [g/L] | — | — |
| Deferoxamine | — | — | 0.025 |
| LK614 | — | — | 0.0075 |
| Calculated osmolarity (mosm/L) | 369 | 311 | 302 |
| Buffer capacity in the pH range 7.0 →6.0 (mmol H+/L): | |||
| At 25°C | 85 | 57 | |
| At 5°C | 97 | 74 | |
HAES = Hydroxyethyl starch; HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution.
|
|
|
Figure 1. Study design from beginning to final evaluation of the transplanted kidneys. HTK-N = new histidine-tryptophan ketoglutarate solution; KTx = porcine kidney transplantation model; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
| Sample | Prereperfusion | Postreperfusion | Postoperative day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Blood | X | X | X | X | |||||
| Biopsy | X | X | X | ||||||
2.4. Preoperative preparation, anesthesia, and cardiovascular monitoring
Preoperative preparation for all animals included fasting for 12 hours, allowing free access to water only, and application of a standardized narcotic protocol with premedication: azaperon 8 mg/kg intramuscular, midazolam 0.5–0.7 mg/kg intramuscular, ketamine 5 mg/kg intravenously (i.v.), and atropine sulfate 1 mg i.v., followed by endotracheal intubation. Pressure controlled ventilation was done in a half-closed system. The ventilation parameters were adjusted to the frequency of 11 minutes, tidal volume of 300 mL, air 1.5–2.0 L/min, O2 0.5–1.0 L/min, N2O 1.5–2.0 L/min, and isoflurane 0.75–1.5%. The arterial blood gases were kept between 75–100 mmHg pO2 and 35–42 mmHg pCO2. Cardiocirculatory invasive measurements included central venous pressure (CVP) and mean arterial pressure (MAP), using an inserted catheter via the internal jugular vein and common carotid artery, respectively.
2.5. Surgical procedures
2.5.1. Explantation and preservation
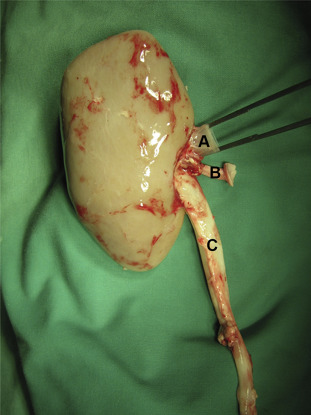
Through a full-length midline laparotomy, kidneys were mobilized and dissected from their perirenal tissue. The vessels were dissected gently. Mobilization of the adrenal gland and division of its vein from the renal vein were followed by ligation and division of the lateral lumbar arteries. After heparinization (25,000 IU) and insertion of the catheter in the aorta abdominalis near the branches of renal arteries, the respective preservation solution was infused with a controlled constant pressure of 120 mmHg and the renal veins were cut to allow emitting the perfusion solution. According to the different application standards, the perfusion volume in the UW group was 500 mL, and perfusion time was 5 minutes. In the standard HTK and HTK-N groups, the perfusion volume was 2 L, and the perfusion took 10 minutes. Kidney explantation was performed with protection of the periureteral fat tissue to preserve blood-vessels of the ureter. Along with the kidney and its respective vessels, the ureter was cut long enough for later ureterocystostomy (Figure 2). After procurement, the organs were kept in the respective 4°C preservation solution for 30 hours of cold ischemia.
|
|
|
Figure 2. Explanted kidney after a 30-hour cold ischemia time [(A) renal vein; (B) renal artery with the carrel patch; and (C) urethra with periurethral tissue].26 |
2.5.2. Implantation
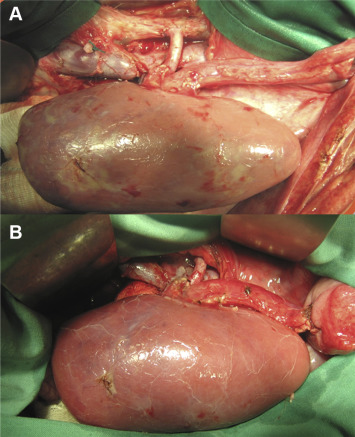
After the 30-hour cold ischemia time and biopsy, the kidneys were transplanted orthotopically in the kidney lodge after both-side nephrectomy of the recipients. The venous anastomosis was constructed end to side with continuous single layer 5/0 prolene-sutures between the renal vein and vena cava inferior. A similar arterial end-to-side running suture anastomosis with 5/0 prolene was performed afterwards between the renal artery and aorta (Figure 3A). After heparinization with 250 IU/kg i.v., the reperfusion started by declamping the renal veins and arteries. Thereafter, the ureter anastomosis was performed using the ureteroneocystostomy technique by Lich-Gregoir with a 4/0 PDS sutures (Figure 3B). During the operation, the recipient received 1–2.5 g metamizole i.v, 100 mL/kg isotonic solution infusion (NaCl 0.9% or Ringer), 2.5 mg/kg i.v enrofloxacin, and 40 mg i.v. pantozol.
|
|
|
Figure 3. Implanted kidney after reperfusion. (A) The arterial and venous anastomosis are completed; (B) transplanted kidney after reperfusion and completed ureterocystostomy.26 |
2.5.3. Peri- and postoperative monitoring
After the operation the permanent central venous catheter was fixed to the subcutaneous tissue of the neck of pigs and the animals were transferred to the universitys animal enclosure and were followed-up under controlled condition for 7 days. They received 250 mg i.v./d methylprednisolone and 0.05 mg/kg i.v./d tacrolimus as imunosupressants, and 0.02 mg/kg i.v./daily buprenorphine and 500 mg/d metamizole as analgesics. To prevent stress ulcers, vascular thrombosis, and infection, pantozol 40 mg i.v./d, enoxaparin 40 mg subcutaneous/d, and enrofloxacin 2.5 mg/kg i.v./d were given, respectively. Mannitol, glucose, and isotonic solution (NaCl) were infused regarding urine output and circulatory stability of the animals (Table 3). Blood samples were taken before and after reperfusion, as well as on Day 3 and Day 7 postoperatively to control serum creatinine, blood urea nitrogen (BUN), hemoglobin (Hb), and hematocrit (Hct; Table 2).
| Analgesia | Metamizol (Novalgin) | 50 mg/kg/d i.v. |
| Pritramid (Dipidolor) | 0.5 mg/kg/d i.v. | |
| Immunosupression | Tacrolimus (Prograf) | 2 g/d i.v. |
| Methylprednisolone (Urbason) | 250 mg/d i.v. | |
| Antibiosis | Meziocilline (Baypen) | 2 g/d i.v. |
| Thrombosis prophylaxis | Enoxaparine (Clexane) | 40 mg/d s.c. |
| Stress ulcer prophylaxis | Pantoprazole (Pantozol) | 40 mg/d i.v. |
| Isotonic fluid | NaCl 0.9% | 1st postoperative d:2 L i.v. |
| 2nd postoperative d: 2 L i.v. |
i.v. = intravenous; s.c. = subcutaneous.
2.5.4. Histopathological assessment
In order to evaluate the histological changes of the transplanted organs, biopsies were taken three times: after cold ischemia time, 30 minutes after reperfusion, and on the 7th posttransplant day (Figure 1). The biopsies were fixed in 5% formaldehyde. The tubular injury was classified in a semiquantitative scoring system for ranking the progress of possible tissue damages (Table 4). According to this scoring system the histological outcome of all three groups at each sampling time was ranked.
| Semiquantitative scoring | |
|---|---|
| Intact tubuli with epithelial brush borders | 0 |
| Focal acute tubular necrosis | 1 |
| Acute tubular necrosis | 2 |
| Focal loss of ciliated borders in epithelial cells | 3 |
| Loss of ciliated borders in epithelial cells | 4 |
| Focal flattening & vacuolization of the epithelial cells | 5 |
| Flattening & vacuolization of the epithelial cells | 6 |
| Focal denudation of basement membrane | 7 |
| Denudation of basement membranes | 8 |
2.6. Statistical analysis
All results are quoted as mean ± standard error of the mean. Statistical analysis was performed using IBM SPSS Statistics, version 22 (Licensed Materials – Property of IBM. © Copyright IBM Corp. 1983, 2013.), using Kruskal–Wallis and analysis of variance tests where appropriate. The null hypothesis was rejected when p < 0.05.
3. Results
3.1. Cardiovascular results
The average weights of the experimental animals between the three recipient groups were not significantly different (30.2 ± 2.7 kg in GI, 30.3 ± 2.8 kg in GII, and 30.2 ± 2.7 kg in GIII). The average operating duration was 215 ± 8 minutes in GI, 210 ± 9 minutes in GII, and 211 ± 8 minutes in GIII. Warm ischemia time in all groups was invariably < 45 minutes. Anastomosing time took ∼35 minutes in each group. Before transplantation, MAP and CVP in GI were 66 ± 2.5 mmHg and 9.6 ± 0.3 cmH2O, 69 ± 2.1 mmHg and 9.3 ± 0.2 cmH2O in GII, and 64 ± 1.9 mmHg and 9.4 ± 0.3 cmH2O in GIII, respectively. After transplantation MAP and CVP were 73 ± 3.1 mmHg and 11.2 ± 1 cmH2O in GI, 77 ± 2.5 mmHg and 12.1 ± 0.8 cmH2O in GII, and 74 ± 2.0 mmHg and 10.9 ± 0.8 cmH2O in GIII, respectively. Neither the differences between the groups nor the differences between pre- and posttransplantation were statistically significant. After transplantation all recipients showed a mild decrease of Hct and Hb. Hct and Hb in GI from 33 ± 3% and 11.35 ± 1.29 g/dL dropped to 31 ± 3% and 11.19 ± 1.63 g/dL, in GII from 31 ± 2% and 10.66 ± 1.11 g/dL to 27 ± 6% and 10.09 ± 1.85 g/dL, and in GIII from 33 ± 2% and 11.86 ± 1.30 g/dL to 31 ± 6% and 11.49 ± 2.78 g/dL. The differences between pre- and posttransplantation, as well as between the three groups were not statistically significant.
3.2. Metabolic studies
3.2.1. General criteria
All implanted kidneys showed a homogenous red color macroscopically within 5 minutes after reperfusion (Figure 3B). The majority of grafts started to excrete urine within the first 24 hours of reperfusion. It happened in 11 of 12 cases in GI, 12 of 12 cases in GII, and 10 of 12 cases in GIII.
3.2.2. Parameters of graft function
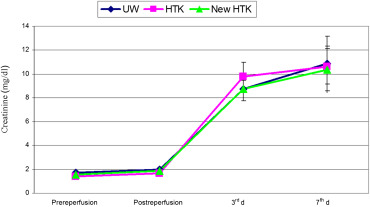
Comparing pre- and postischemic serum potassium concentration showed a postischemic increase in all graft recipients up to pathological values. This increase was minor in the groups UW and standard HTK, but obvious in the HTK-N group. The values, however, were highly variable and consequently not significantly different. Serum creatinine in donors were 1.7 ± 0.3 mg/dL in GI, 1.45 ± 0.3 mg/dL in GII, and 1.54 ± 0.3 mg/dL in GIII. The differences between these values were not statistically significant. During the first 3 days after transplantation in all three groups, creatinine increased to pathological values. This increase was minor in the HTK-N group as compared with the other groups, but reached no statistical significance. In Group I, it reached 9.6 ± 2.3 mg/dL, in Group II 10.5 ± 4.25 mg/dL, and in Group III 8.5 ± 4.8 mg/dL (Figure 4).
|
|
|
Figure 4. Mean values and standard deviations of serum creatinin in three groups of UW, standard HTK, and HTK-N during the experimental study (pre-, postreperfusion, 3rd day and 7th day after transplantation). HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
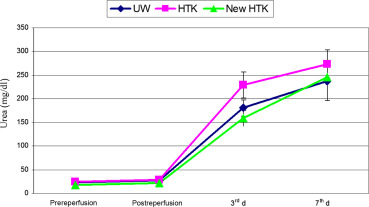
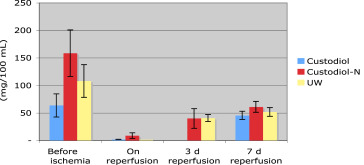
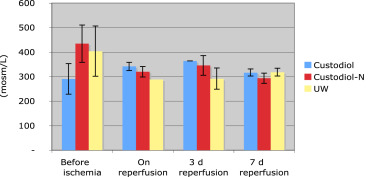
BUN also increased during the first 3 days postoperation to the pathological values in all three groups. BUN during this interval rose from 23 ± 6 mg/dL to 240 ± 125 mg/dL in Group I, and from 22 ± 9 mg/dL to 251 ± 104 mg/dL in Group II. Neither before implantation nor on Day 3 postoperation, the values differed significantly. The data are summarized in Figure 5. In urine, creatinine concentration in the three experimental groups was higher preischemically than postischemically. During reperfusion, it increased to around 50 mg/100 mL in all three groups. This increase was independent of the preservation used and statistically not different (Figure 6). The levels of urine osmolarity in different time points were comparable in all groups (Figure 7).
|
|
|
Figure 5. Mean values and standard deviations of serum urea in three groups of UW, standard HTK, and HTK-N during the experimental study (pre-, postreperfusion, 3rd day and 7th day after transplantation). HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
|
|
|
Figure 6. Mean values and standard error of mean of urine creatinine (mg/100 mL) in three groups of UW, standard HTK, and HTK-N, during the experimental study (pre-, postreperfusion, 3rd day and 7th day after transplantation). HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
|
|
|
Figure 7. Mean values and standard error of mean of urine osmolarity (mosm/L) in three groups of UW, standard HTK, and HTK-N during the experimental study (pre-, postreperfusion, 3rd day and 7th day after transplantation). |
3.3. Histopathological results
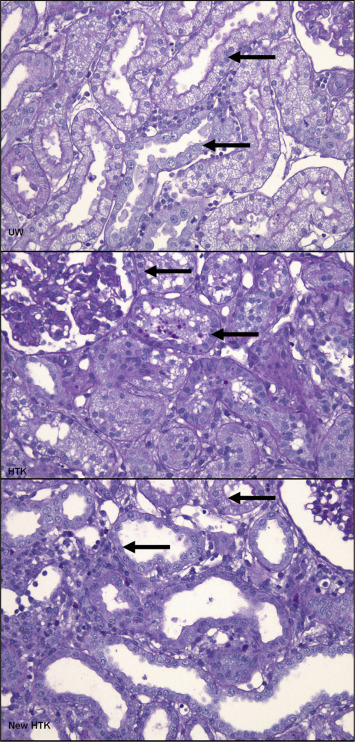
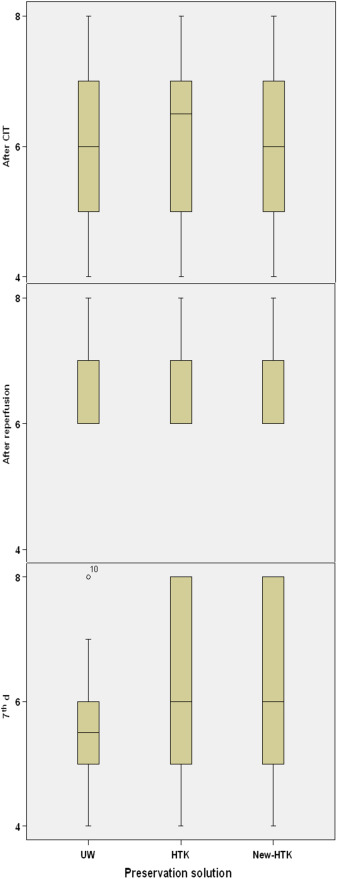
The biopsies were evaluated for tubular injury and nekrosis. The tubular injury was classified based on the semi quantitative scoring system shown in Table 4. One hour after reperfusion, histopathological deterioration could be seen in 13% of kidneys preserved by UW and standard HTK, and in 15% preserved by HTK-N. On posttransplant Day 7, we could see the sign of regeneration in all kidneys. When classifying the extent of this regeneration into A ≥ 75%, B 25–75%, C < 25%, UW kidneys showed 70%, HTK 80%, and HTK-N 70% of Level A regeneration. Level B was seen in about 30% of UW Kidneys, in 10% of standard HTK kidneys, and in 20% of HTK-N kidneys. Level C could be seen in none of the UW kidneys, 10% of standard HTK, and 20% of HTK-N kidneys. Considering the extent of the regeneration on Day 7 posttransplant, UW, standard HTK, and HTK-N reached 16%, 12%, and 10% histopathological improvement, respectively (Figure 8). Neither deterioration nor improvement of the histopathological structure showed significant difference between the solutions in each biopsy phase (after cold ischemia time: p = 0.9; 1 hour after reperfusion: p = 0.8; 7th day: p = 0.5; Figure 9).
|
|
|
Figure 8. Samples of the microscopic view from the histopathological results of the transplanted kidneys at 7th postoperative day. All biopsies were stained with Gimsa stain. The nucleoli in regenerating epithelial cells are seen in all pictures (arrows). HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
|
|
|
Figure 9. Comparison of the histopathological results based on the semi quantitative scores through Kuruskal–Wallis test. The vertical line is the calculated score regarding the semiquantitative scoring system. The rectangles depict the range between the lower (25%) and upper (75%) quarter of the evaluation. The median is represented by the horizontal line in the middle of the rectangles. CIT = cold ischemia time; HTK-N = new histidine-tryptophan ketoglutarate solution; standard HTK = standard histidine-tryptophan ketoglutarate solution; UW = University of Wisconsin solution. |
4. Discussion
The aim of optimizing graft quality has resulted in intensive experimental and clinical efforts to improve the ischemic tolerance of the organs. One of the most important goals is to improve the preserving quality of the current preservation solutions or to invent new ones. In our study, we have compared HTK-N with the two clinically established preservation solutions (UW and standard HTK) in a porcine model of KTx. Due to a physiological and anatomical resemblance between pig and humans, a porcine model has been considered suitable for experimental studies of kidney transplantation.24; 25; 26 ; 27
There is a close correlation between histological changes and the postischemic organ situation.28 ; 29 As the proximal tubule considered being the most sensitive part against ischemic injury,30 ; 31 we have evaluated these injuries based on a semiquantitative scoring system of acute tubular injury and compared the histopathological changes of the transplanted organs in each group during 7 days follow-up. The results showed deterioration of the scores in each group after reperfusion parallel with a rise in BUN and creatinine values without any significant difference. Furthermore, the regeneration of the proximal tubule after kidney transplantation can be considered as the crucial step towards the restoration of renal function.22 The more complete the regeneration is, the better prognosis for the kidney function is expected.32 ; 33 In our study also, on the 7th postoperative day, compared with the 3rd day, we could achieve better scores. Simultaneous comparison of the results of BUN and creatinine also shows a milder rise of the values between 3rd day and 7th day in comparison to the first 3 posttransplant days. Based on the histopathological results, HTK-N solution depicted comparable results with UW and standard HTK without any significant difference.
In our study, in the pig kidneys transplanted after 30 hours of cold ischemia, according to clinical standards the new HTK-N solution seems to be as protective as the standard HTK and UW used in many transplantation centers world-wide.34; 35; 36; 37; 38 ; 39 This result, however, is not consistent with recent findings in the literature for HTK-N. There are several reports on a significant gain in protective efficacy in rat liver cell and liver ischemia-reperfusion models.15; 40 ; 41 Moreover, positive results are published in rat liver endothelial cells and isolated hepatocytes.17; 18; 42 ; 43 Even in rat heart ischemia-reperfusion studies, HTK-N is evaluated with positive results.14; 44; 45; 46 ; 47 Recently positive preservation results were also described in rat and human arteries.13; 18 ; 48 Although the experimental models used in these papers are not completely comparable with patients, there are a number of reports in favor of HTK-N as compared with standard HTK or UW.
The HTK-N solution differs from standard HTK in some points, which are actually discussed to be decisive for the limit of ischemia tolerance in solid organs and the degree of ischemia-reperfusion injury.2; 16 ; 49 The main points are: (1) its higher antioxidative capacity; (2) its reduced histidine content; (3) its higher magnesium and calcium content; and (4) its lower chloride and the replacement of mannitol by raffinose as osmolyte (Table 1). The most important innovative difference is that HTK-N is supplemented by the synthetic iron-catalase-mimic LK614 and the iron complexing deferoxamine.50 LK614 is classified as a cell-permeable scavenger of free reactive oxygen species, so-called free radicals. Free radicals in aerobiosis have significant functions in cell signaling.51 ; 52 In the reperfusion phase after a prolonged ischemic period, they are one of the main causes of the so-called ischemia-reperfusion injury. They are able to oxidize and impair nearly every cell component by their high oxidation potential.2; 16; 49; 50; 51; 52; 53 ; 54 Deferoxamine, a membrane permeable iron complex, is working in the same direction. Under physiologic conditions, the fraction of free iron in cells is kept extremely low.51 ; 55 It increases, however, e.g., late in ischemia-caused acidosis and/or in destruction of mitochondrial membranes. In these conditions, it reacts with H2O2, a side-product and regulator of cell metabolism, forming the especially aggressive hydroxyl radicals. This happens via a Fenton reaction, which can work like a vicious cycle and thereby potentiates the problem of free radical injury. Additional free iron in the HTK-N solution should work as a further antioxidant besides LK614.43 ; 50
A further central modification in HTK-N may be the reduction of the histidine buffer by about one-third in favor of the addition of acetyl-histidine. The hypothesis behind this is a possible toxic effect of products of histidine oxidation.42 This modification, however, also reduces the buffer capacity of HTK-N compared with Custodiol in the range of pH 7.0 to 6.0, which typically is found in long-lasting ischemic periods.56 ; 57 This results from the pKs value of acetyl-histidine which at 20°C is 7.25 and 0.3–0.4 units higher at 5°C. But the relevancy of this effect remains to become clarified. Next, the magnesium concentration in HTK-N has been increased from 4 mmol/L to 8 mmol/L. This approach is known to be very protective especially during ischemia, as shown in the early version of the standard HTK solution.58 Magnesium in several respects works like a calcium-antagonistic drug. High magnesium in dog hearts after warm ischemia and reperfusion was shown to result in reperfusion arrhythmias, which became severe when HTK was reapplied during an ischemic period.58 ; 59 These arrhythmias were a form of calcium paradox caused by calcium depletion of the cellular glycocalyx and could be relieved by the reduction of magnesium or calcium supplementation.23; 57; 58 ; 59 From this point of view it is consequent, which besides 8mM magnesium in HTK-N contains more calcium than Custodiol or UW. Finally, in HTK-N, chloride concentration is reduced from 50 mmol/L to 30 mmol/L and mannitol is substituted by raffinose, an effective way to reduce the risk of cell swelling by the solution. This may be a main issue in liver preservation, because hepatocytes are permeable for manitol but impermeable for raffinose.
In summary, HTK-N should improve the preservation efficiency by reducing the risk of uncontrolled free radicals, possible toxicity of degradation products of histidine, fast energy deficit, and cell edema. Therefore, we had expected to find real functional or pathological advantages in pig kidney preservation and transplantation. Certainly, results in cell culture or in isolated rat organs are far from big animal or patient conditions.53; 60; 61 ; 62 Nevertheless, an advantage of HTK-N compared with the standard solutions such as Custodiol and UW may become measurable in clinical setting, where additional factors such as old-for-old organ, extremely long ischemia time, or marginal organs require specific qualities of a preservation solution. Hence, it should be justifiable to start such studies in the view of the experimental results obtained in the pig model of kidney transplantation shown here.
References
- 1 F.O. Belzer, J.H. Southard; Principles of solid-organ preservation by cold storage; Transplantation, 45 (1988), pp. 673–676
- 2 H. de Groot, U. Rauen; Ischemia-reperfusion injury: processes in pathogenetic networks: a review; Transplant Proc, 39 (2007), pp. 481–484
- 3 R.F. Parsons, J.V. Guarrera; Preservation solutions for static cold storage of abdominal allografts: which is best?; Curr Opin Organ Transplant, 19 (2014), pp. 100–107
- 4 G. Gubernatis, R. Pichlmayr, P. Lamesch, et al.; HTK-solution (Bretschneider) for human liver transplantation. First clinical experiences; Langenbecks Arch Chir, 375 (1990), pp. 66–70
- 5 G. den Butter, A. Saunder, D.C. Marsh, F.O. Belzer, J.H. Southard; Comparison of solutions for preservation of the rabbit liver as tested by isolated perfusion; Transpl Int, 8 (1995), pp. 466–471
- 6 A. Aminalai, G. Kehrer, F. Grossmann, J. Richter, H.J. Bretschneider; Morphological investigation of the porcine liver directly following preservation with Euro-Collins, University of Wisconsin and Bretschneiders HTK solution; Langenbecks Arch Chir, 377 (1992), pp. 81–88
- 7 L. Roels, W. Coosemans, J. Donck, et al.; Inferior outcome of cadaveric kidneys preserved for more than 24 hr in histidine-tryptophan-ketoglutarate solution. Leuven Collaborative Group for Transplantation; Transplantation, 66 (1998), pp. 1660–1664
- 8 H. Schneeberger, S. Schleibner, W.D. Illner, K. Messmer, W. Land; The impact of free radical-mediated reperfusion injury on acute and chronic rejection events following cadaveric renal transplantation; Clin Transpl (1993), pp. 219–232
- 9 W.C. Goggins, M.A. Pascual, J.A. Powelson, et al.; A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients; Transplantation, 76 (2003), pp. 798–802
- 10 A. Beiras-Fernandez, E. Thein, D. Chappel, et al.; Polyclonal anti-thymocyte globulins influence apoptosis in reperfused tissues after ischaemia in a non-human primate model; Transpl Int, 17 (2004), pp. 453–457
- 11 D. Bogetti, H.N. Sankary, T.M. Jarzembowski, et al.; Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury; Clin Transpl, 19 (2005), pp. 507–511
- 12 J.A. Akoh; Kidney donation after cardiac death; World J Nephrol, 1 (2012), pp. 79–91
- 13 B. Zatschler, P. Dieterich, B. Muller, M. Kasper, U. Rauen, A. Deussen; Improved vessel preservation after 4 days of cold storage: experimental study in rat arteries; J Vasc Surg, 50 (2009), pp. 397–406
- 14 A. Koch, S. Loganathan, T. Radovits, F.U. Sack, M. Karck, G.B. Szabo; Deferoxamine, the newly developed iron chelator LK-614 and N-alpha-acetyl-histidine in myocardial protection; Interact Cardiovasc Thorac Surg, 10 (2010), pp. 181–184
- 15 R. Bahde, D. Palmes, O. Gemsa, et al.; Attenuated cold storage injury of rat livers using a modified HTK solution; J Surg Res, 146 (2008), pp. 49–56
- 16 E.J. Lesnefsky, K.G. Allen, F.P. Carrea, L.D. Horwitz; Iron-catalyzed reactions cause lipid peroxidation in the intact heart; J Mol Cell Cardiol, 24 (1992), pp. 1031–1038
- 17 U. Rauen, H. de Groot; Inherent toxicity of organ preservation solutions to cultured hepatocytes; Cryobiology, 56 (2008), pp. 88–92
- 18 C. Schroder, A. Heintz, A. Pexa, U. Rauen, A. Deussen; Preclinical evaluation of coronary vascular function after cardioplegia with HTK and different antioxidant additives; Eur J Cardiothorac Surg, 31 (2007), pp. 821–826
- 19 B.C. White, G.S. Krause, S.D. Aust, G.E. Eyster; Postischemic tissue injury by iron-mediated free radical lipid peroxidation; Ann Emerg Med, 14 (1985), pp. 804–809
- 20 N. Tapuria, Y. Kumar, M.M. Habib, M. Abu Amara, A.M. Seifalian, B.R. Davidson; Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury–a review; J Surg Res, 150 (2008), pp. 304–330
- 21 K. Kloppel, J. Gerlach, P. Neuhaus; The electrolyte composition of liver preservation solutions for hepatocytes in a model of in vitro preservation and reoxygenation; Langenbecks Arch Chir, 379 (1994), pp. 210–217
- 22 K. Umeshita, M. Monden, T. Fujimori, et al.; Extracellular calcium protects cultured rat hepatocytes from injury caused by hypothermic preservation; Cryobiology, 25 (1988), pp. 102–109
- 23 M.M. Gebhard, W. Gross, M. Schäfer; Organprotektion: Neue aspekte; Chirurgische Forschung (2005), pp. 317–324 [in German]
- 24 S. Zonta, F. Lovisetto, C. Lorenzo, et al.; Uretero-neocystostomy in a swine model of kidney transplantation: a new technique; J Surg Res, 124 (2005), pp. 250–255
- 25 A. Wu, K. Yamada, F.L. Ierino, P.A. Vagefi, D.H. Sachs; Regulatory mechanism of peripheral tolerance: in vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine; Transpl Immunol, 11 (2003), pp. 367–374
- 26 M. Golriz, H. Fonouni, A. Nickkholgh, M. Hafezi, C. Garoussi, A. Mehrabi; Pig kidney transplantation: an up-to-date guideline; Eur Surg Res, 49 (2012), pp. 121–129
- 27 M. Golriz, M. Hafezi, C. Garoussi, et al.; Do we need animal hands-on courses for transplantation surgery?; Clin Transpl, 27 (2013), pp. S6–15
- 28 M.H. Booster, G.J. van der Vusse, R.M. Wijnen, M. Yin, B.M. Stubenitsky, G. Kootstra; University of Wisconsin solution is superior to histidine tryptophan ketoglutarate for preservation of ischemically damaged kidneys; Transplantation, 58 (1994), pp. 979–984
- 29 H.J. Bretschneider, U. Helmchen, G. Kehrer; Kidney protection; Klin Wochenschr, 66 (1988), pp. 817–827
- 30 M. Busing, U.T. Hopt, W. Schareck, M. Ernst, K. Morgenroth; Ultrastructural changes of different preserved human kidney allografts before and after reperfusion; Transplant Proc, 22 (1990), pp. 448–449
- 31 J.K. Orak, A.K. Singh, P.R. Rajagopalan, I. Singh; Morphological analysis of mitochondrial integrity in prolonged cold renal ischemia utilizing Euro-Collins versus University of Wisconsin preservation solution in a whole organ model; Transplant Proc, 26 (1994), pp. 122–125
- 32 O. Kwon, W.J. Nelson, R. Sibley, et al.; Backleak, tight junctions, and cell- cell adhesion in postischemic injury to the renal allograft; J Clin Invest, 101 (1998), pp. 2054–2064
- 33 M. Franquesa, M. Flaquer, J.M. Cruzado, J.M. Grinyo; Kidney regeneration and repair after transplantation; Curr Opin Organ Transplant, 18 (2013), pp. 191–196
- 34 J. de Boer, J. De Meester, J.M. Smits, et al.; Eurotransplant randomized multicenter kidney graft preservation study comparing HTK with UW and Euro-Collins; Transpl Int, 12 (1999), pp. 447–453
- 35 J. Erhard, R. Lange, R. Scherer, et al.; Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study; Transpl Int, 7 (1994), pp. 177–181
- 36 L. Feng, N. Zhao, X. Yao, et al.; Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review; Liver Transpl, 13 (2007), pp. 1125–1136
- 37 J.A. Fridell, R.S. Mangus, A.J. Tector; Clinical experience with histidine-tryptophan-ketoglutarate solution in abdominal organ preservation: a review of recent literature; Clin Transpl, 23 (2009), pp. 305–312
- 38 R.S. Mangus, A.J. Tector, J.A. Fridell, M. Kazimi, E. Hollinger, R.M. Vianna; Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in intestinal and multivisceral transplantation; Transplantation, 86 (2008), pp. 298–302
- 39 R. Montalti, B. Nardo, E. Capocasale, et al.; Kidney transplantation from elderly donors: a prospective randomized study comparing celsior and UW solutions; Transplant Proc, 37 (2005), pp. 2454–2455
- 40 U. Kerkweg, M. Jacob, H. De Groot, H.G. Mannherz, U. Rauen; Cold-induced apoptosis of rat liver endothelial cells: contribution of mitochondrial alterations; Transplantation, 76 (2003), pp. 501–508
- 41 S. Wu, J. Wohlschlaeger, H. de Groot, U. Rauen; Evaluation of a modified HTK solution containing the new iron chelator LK 614 in an isolated rat liver perfusion model; J Invest Surg, 22 (2009), pp. 340–347
- 42 U. Rauen, S. Klempt, H. de Groot; Histidine-induced injury to cultured liver cells, effects of histidine derivatives and of iron chelators; Cell Mol Life Sci, 64 (2007), pp. 192–205
- 43 U. Rauen, F. Petrat, R. Sustmann, H. de Groot; Iron-induced mitochondrial permeability transition in cultured hepatocytes; J Hepatol, 40 (2004), pp. 607–615
- 44 A. Koch, T. Radovits, S. Loganathan, F.U. Sack, M. Karck, G.B. Szabo; Myocardial protection with the use of L-arginine and N-alpha-acetyl-histidine; Transplant Proc, 41 (2009), pp. 2592–2594
- 45 S. Loganathan, T. Radovits, K. Hirschberg, et al.; Effects of Custodiol-N, a novel organ preservation solution, on ischemia/reperfusion injury; J Thorac Cardiovasc Surg, 139 (2010), pp. 1048–1056
- 46 T. Radovits, L.N. Lin, J. Zotkina, et al.; Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: effects of iron chelators and N-alpha-acetyl-L-histidine; J Heart Lung Transplant, 27 (2008), pp. 208–216
- 47 K. Wu, T.R. Turk, U. Rauen, et al.; Prolonged cold storage using a new histidine-tryptophan-ketoglutarate-based preservation solution in isogeneic cardiac mouse grafts; Eur Heart J, 32 (2011), pp. 509–516
- 48 S. Garbe, B. Zatschler, B. Muller, et al.; Preservation of human artery function following prolonged cold storage with a new solution; J Vasc Surg, 53 (2011), pp. 1063–1070
- 49 M.D. Menger, B. Vollmar; Pathomechanisms of ischemia-reperfusion injury as the basis for novel preventive strategies: is it time for the introduction of pleiotropic compounds?; Transplant Proc, 39 (2007), pp. 485–488
- 50 U. Rauen, T. Li, R. Sustmann, H. De Groot; Protection against iron- and hydrogen peroxide-dependent cell injuries by a novel synthetic iron catalase mimic and its precursor, the iron-free ligand; Free Radic Biol Med, 37 (2004), pp. 1369–1383
- 51 P. Arosio, S. Levi; Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage; Biochim Biophys Acta, 1800 (2010), pp. 783–792
- 52 K. Nowak, C. Hanusch, K. Nicksch, et al.; Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies; Eur J Cardiothorac Surg, 37 (2010), pp. 859–863
- 53 J.M. Gutteridge; Does redox regulation of cell function explain why antioxidants perform so poorly as therapeutic agents?; Redox Rep, 4 (1999), pp. 129–131
- 54 O.I. Aruoma, M. Grootveld, T. Bahorun; Free radicals in biology and medicine: from inflammation to biotechnology; Biofactors, 27 (2006), pp. 1–3
- 55 A.C. Chua, R.M. Graham, D. Trinder, J.K. Olynyk; The regulation of cellular iron metabolism; Crit Rev Clin Lab Sci, 44 (2007), pp. 413–459
- 56 M.M. Gebhard; Pathophysiology of global ischemia of the heart; Z Kardiol, 76 (1987), pp. S115–129
- 57 M.M. Gebhard, H.J. Bretschneider, E. Gersing, C.J. Preusse, P.A. Schnabel, L.J. Ulbricht; Calcium-free cardioplegia–pro; Eur Heart J, 4 (1983), pp. S151–160
- 58 M.M. Gebhard, C.J. Preusse, P.A. Schnabel, H.J. Bretschneider; Different effects of cardioplegic solution HTK during single or intermittent administration; Thorac Cardiovasc Surg, 32 (1984), pp. 271–276
- 59 M.M. Gebhard, E. Gersing, C.J. Brockhoff, P.A. Schnabel, H.J. Bretschneider; Impedance spectroscopy: a method for surveillance of ischemia tolerance of the heart; Thorac Cardiovasc Surg, 35 (1987), pp. 26–32
- 60 B. Halliwell; Biochemistry of oxidative stress; Biochem Soc Trans, 35 (2007), pp. 1147–1150
- 61 L.H. Long, A. Hoi, B. Halliwell; Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media; Arch Biochem Biophys, 501 (2010), pp. 162–169
- 62 T. Radovits, J. Zotkina, L.N. Lin, M. Karck, G. Szabo; Endothelial dysfunction after hypoxia-reoxygenation: do in vitro models work?; Vascul Pharmacol, 51 (2009), pp. 37–43
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?