Abstract
Aims
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy, diastolic dysfunction and increased interstitial fibrosis. Current treatment is based on beta-adrenoceptor (AR) and calcium channel blockers. Since mice deficient of protein phosphatase-1 inhibitor-1 (I-1), an amplifier in beta-AR signalling, were protected from pathological adrenergic stimulation in vivo, we hypothesized that I-1 ablation could result in an improved outcome in a HCM mouse model.
Methods and results
We crossed mice deficient of I-1 with homozygous myosin-binding protein C knock-out (Mybpc3 KO) mice exhibiting cardiac dilatation and reduced survival. Unexpectedly, survival time was shorter in double I-1/Mybpc3 KO than in single Mybpc3 KO mice. Longitudinal echocardiographic assessment revealed lower fractional area change, and higher diastolic left ventricular inner dimensions and end-diastolic volumes in Mybpc3 KO than in WT mice. In comparison to Mybpc3 KO, double I-1/Mybpc3 KO presented higher left ventricular end-diastolic volumes, inner dimensions and ventricular surface areas with increasing differences over time. Phosphorylation levels of PKA-downstream targets and mRNA levels of hypertrophic markers did not differ between I-1/Mybpc3 KO and single Mybpc3 KO mice, except a trend towards higher beta-myosin heavy chain levels in double I-1/Mybpc3 KO.
Conclusion
The data indicate that interference with beta-AR signalling has no long-term benefit in this severe MYBPC3-related cardiomyopathy mouse model.
Keywords
Beta-adrenergic signalling;Cardiomyopathy;Hypertrophy;Hypertrophic cardiomyopathy
1. Introduction
Hypertrophic cardiomyopathy (HCM) is mainly characterized by asymmetric left ventricular hypertrophy, diastolic dysfunction and myocardial disarray [1]. To date, HCM is known to be caused by mutations in at least 23 different genes encoding mainly sarcomeric proteins [2]. An early concept of HCM is a hyper-adrenergic state [3]; [4]; [5] ; [6], which is a rationale for beta-adrenoceptor (AR) blockade as treatment option for HCM in humans. Beta-AR blocker treatment has proven to be beneficial in patients with angina or dyspnea on exertion, especially when associated with left ventricular outflow tract (LVOT) obstruction, and is frequently used to lower the occurrence of non-sustained ventricular arrhythmias in HCM patients [7]; [8] ; [9]. On the cellular level, beta-AR blockade leads to lower cyclic AMP production. In pacemaker cells, this lowers spontaneous beating activity with the consequence of a lower heart rate, whereas in ventricular myocytes, protein kinase A (PKA) activity is alleviated, leading to a lower phosphorylation status of L-type Ca2 + channels (LTCC), phospholamban (PLB), cardiac troponin I (cTnI), cardiac myosin-binding protein C (cMybpc) and cardiac ryanodine receptors (RyR2).
A downstream element of the cardiac beta-AR signalling pathway is phosphatase-1 inhibitor-1 (I-1). I-1 inhibits protein phosphatase type-1 (PP1), the major isoform of Ser-Thr-protein phosphatases in the heart, and thereby enhances PKA-mediated protein phosphorylation [10]; [11] ; [12]. In adult rat and in failing human cardiac myocytes, overexpression of wild-type (WT) I-1 or a constitutively active I-1 (I-1c) mutant protein enhanced beta-AR/cAMP/PKA-dependent contractile responses, accentuating its amplifier role in the beta-AR signalling pathway [5] ; [13]. In addition, inducible cardiac I-1c expression in adult mice protected against ischemia–reperfusion-injury and was connected with smaller infarct size and apoptotic injuries [14]. Recently, a study reported that I-1c overexpression with adeno-associated virus (AAV) improved cardiac function in a pig model of ischemic heart failure [15]. In contrast, in a chronic isoprenaline infusion model, I-1 overexpression induced detrimental effects, whereas I-1 deficiency was associated with beneficial effects [16]. These effects were associated with lower steady-state phosphorylation levels of both RyR2 and PLB, without an influence on basal cardiac function, contractile reserve, phosphorylation status of LTCC and key myofibrillar proteins suggesting that PP1 and I-1 strongly control RyR2 and PLB, apparently more than other PKA targets. The data indicate that under conditions of increased adrenergic drive the absence of I-1 is cardioprotective due to the lack of the I-1-mediated intracellular amplification loop. It was therefore suggested that I-1 deficiency acts as an ‘internal beta-blocker’ without influence on heart rate and phosphorylation of LTCC and myofilament proteins [17].
Here, we tested the hypothesis that this I-1 deficiency profile would have also a beneficial effect in a mouse model of HCM. We crossed I-1 knock-out (KO) mice with a HCM Mybpc3 KO mouse model and assessed the effect of I-1 deficiency on prognosis and cardiac function. For comparison, we treated Mybpc3 knock-in mice (KI), another HCM model with more similarity to human HCM [18], chronically with metoprolol. In contrast to our hypothesis, we observed higher mortality combined with worse functional parameters in double I-1/Mybpc3 KO (DKO) than in single Mybpc3 KO (SKO) mice and no apparent beneficial effect of metoprolol on KI mice.
2. Materials and methods
2.1. Experimental animals and survival curve
The study complies with the Guide for the Care and Use of Laboratory Animals published by the NIH (Publication No. 85-23, revised 1985). Mice were handled and maintained according to approved protocols of the animal welfare committee of the University of Hamburg. For establishing the DKO mouse line, homozygous SKO mice [19]; [20] ; [21] were crossed with I-1 KO mice [22]. Mice were maintained on the C57/BL6J genetic background. For the survival curve, 61 DKO, 58 SKO and 22 WT mice were included. Mybpc3-targeted knock-in (KI) mice were developed previously and maintained on the Black Swiss genetic background [18].
2.2. Transthoracic echocardiography
For the longitudinal study with DKO and SKO mice, cardiac functional parameters of 6 mice per group (WT, DKO, SKO) were analyzed by transthoracic echocardiography at 7, 13, 20, 25 and 32 weeks of age using the Vevo 770™ high resolution imaging system (Visual Sonics Inc., Toronto, Canada) as previously described [23].
2.3. Long-term metoprolol treatment and echocardiography
Groups of 10 Mybpc3 WT or KI mice received either drinking water without (control group) or with metoprolol (treatment group) starting at the age of 6–8 weeks for a period of 6 months. Based on water consumption, mice were dosed with 100 mg/kg/day of metoprolol.
Echocardiography was performed every 8–9 weeks using the Vevo 2100 System (VisualSonics, Toronto, Canada). The last echo was performed after 6 months of treatment. Then animals were killed by cervical dislocation and body parameters were obtained.
2.4. Expression analysis
For molecular biology analysis, 34–35-week old WT, SKO and DKO mice were sacrificed by cervical dislocation; hearts were extracted and frozen in liquid-nitrogen cooled isopentane for subsequent molecular-biological analysis. RNA was isolated from powdered mouse ventricular samples using the SV Total RNA Isolation kit (Promega) and 200 ng transcribed into cDNA using the SuperScript® III Reverse Transcriptase kit (Life Technologies) [24] ; [25]. Quantitative determination of atrial natriuretic peptide (Nppa), brain natriuretic peptide (Nppb), α-skeletal actin (Acta1) and beta-myosin heavy chain (Myh7) mRNA levels was performed by real-time PCR using the Maxima SYBR Green/Rox qPCR Master Mix (Thermo Scientific) and primers specific for every sequence (Supplemental Table 1). Ct values were normalized to G alpha s (Gαs). ∆∆Ct values were related to WT.
Western blotting was performed as described previously [13] ; [26]. Details on primary antibodies are provided in Supplemental Table 2.
2.5. Statistical analysis
Data are reported as mean ± S.E.M. Statistical differences between the groups of mice were either calculated by one-way or two-way ANOVA as indicated in the figure legends, using the GraphPad software (GraphPad Software Inc.), version 5.02. Survival analysis of DKO vs. SKO was calculated by the Kaplan–Meier method. A value of p < 0.05 was considered significant.
3. Results
3.1. I-1-deficiency in a Mybpc3 KO mouse model impacts negatively on survival
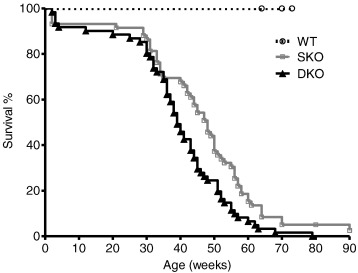
For long term evaluation of I-1-deficiency in the Mybpc3 KO mouse model we performed a survival study with homozygous SKO, DKO and WT mice. Both DKO and SKO had shorter survival rates than WT mice ( Fig. 1). Unexpectedly, DKO presented a significantly shorter median survival than SKO mice (39 vs. 48 weeks, p < 0.05), despite unchanged survival rates of single I-1 deficient mice compared to WT mice (data not shown). There was no gender difference (data not shown). None of the WT mice died during the study period.
|
|
|
Fig. 1. Analysis of survival of WT, single Mybpc3 KO (SKO) and double I-1/Mybpc3 KO (DKO) mice. Kaplan–Meier cumulative survival curves of wild type (WT), single Mybpc3 KO (SKO) and double I-1/Mybpc3 KO (DKO) mice from birth on. Median survival rates were: SKO = 48 weeks, DKO = 39 weeks, log-rank (Mantel–Cox) test, p < 0.001 vs. WT for SKO/DKO, p < 0.05 vs. SKO. None of the WT mice died during the study period. |
This outcome suggests that I-1-deficiency is not beneficial in this Mybpc3 KO mouse model of severe HCM.
3.2. DKO mice show larger ventricles and higher diastolic volume than SKO mice
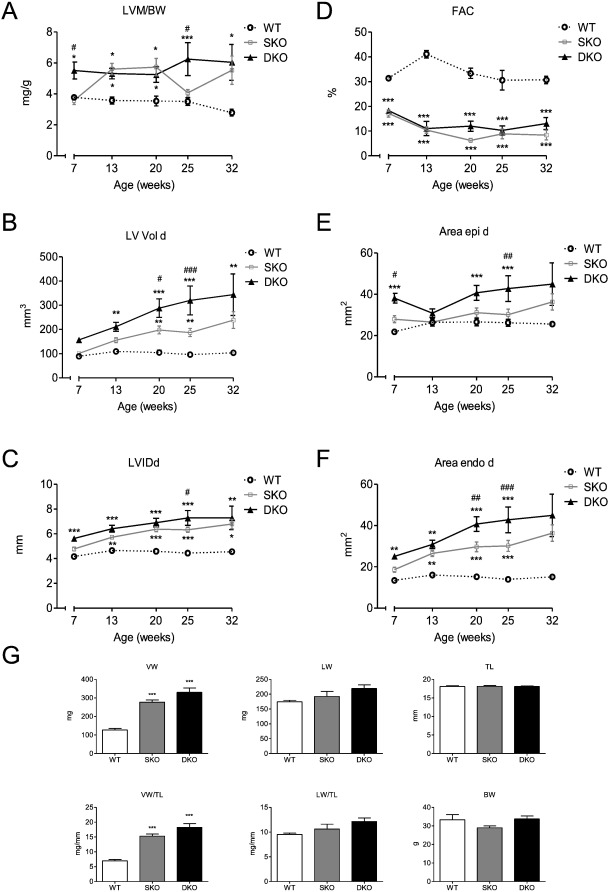
To investigate why I-1 deficiency impacts negatively on survival in our model we performed a longitudinal echocardiography study on animals of each genotype (7 until 32 weeks of age). Echo analysis over the course of time revealed no difference in fractional area change (FAC) at the different ages between SKO and DKO (both were markedly reduced compared to WT), but a higher left ventricular mass to body weight ratio (LVM/BW) for DKO than SKO mice at the age of 7 and 25 weeks, but no difference at 32 weeks of age (Fig. 2A, D).Furthermore, left ventricular end-diastolic volume (LV Vold) and left ventricular inner dimensions in diastole (LVIDd) were higher in DKO than in SKO mice. This difference increased over the course of time, illustrating a dilated phenotype with increased volume retention (Fig. 2B, C). External and internal left ventricular areas in diastole (Area epi/endo d) were also higher in DKO mice, supporting the observations of higher chamber dimensions and a more pronounced dilation phenotype than in SKO (Fig. 2E, F). Since the median survival of DKO mice was 39 weeks (Fig. 1), we assessed ventricular, lung and body weight parameters in 34–35-week old mice of all genotypes. Body and lung weights did not differ between the different genotypes (Fig. 2G). SKO and DKO mice showed a cardiomyopathic phenotype with higher ventricular weight (VW) and ventricular weight to tibia length ratios (LV/TL) than the WT. Ventricular and lung parameters (VW, VW/TL, LW, LW/TL) showed a tendency to slightly higher values in DKO than in SKO mice.
|
|
|
Fig. 2. Longitudinal echocardiography study and body parameters of WT, SKO and DKO mice. A, Left ventricular mass/body weight ratio [LVM/BW], B, fractional area change [FAC], C, left ventricular enddiastolic volume [LV Vol d], D, left ventricular internal enddiastolic diameter [LVIDd], E and F, external (Area epi d) and internal (Area endo d) left ventricular enddiastolic areas were measured in WT, SKO and DKO, n = 6. G, ventricular weight (VW), lung weight (LW), tibia length (TL), ventricular weight/tibia length ratio (VW/TL), lung weight/tibia length ratio (LW/TL) and body weight (BW) were assessed in 34–35-week old WT, SKO and DKO mice, n = 6–9. Data are expressed as mean ± SEM, *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT, #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. SKO, two-way ANOVA followed by Bonferroni comparison post-test or one-way ANOVA followed by Dunnetts comparison post-test. |
3.3. No difference in levels of hypertrophic markers and calcium-handling proteins between DKO and SKO
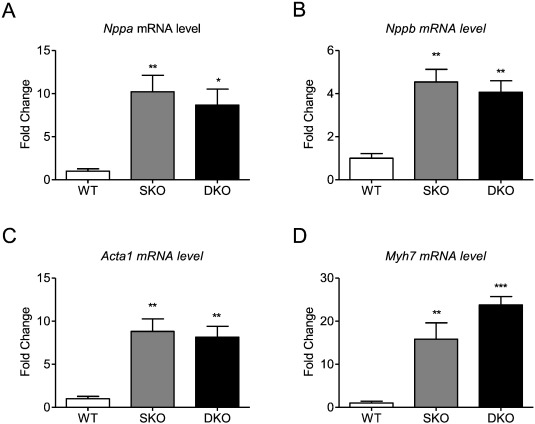
Transcript levels of the hypertrophic markers Nppa, Nppb, Acta1, and Myh7 were markedly higher in SKO and DKO than in WT, confirming the disease phenotype, but did not differ between SKO and DKO which is in line with unchanged Nppa and Nppb levels between I-1 deficient and WT mice [16]. An exception could be detected for Myh7 levels that were higher by trend in DKO ( Fig. 3A–D).
|
|
|
Fig. 3. Expression of markers of the hypertrophic gene program. mRNA levels of A, Nppa, B, Nppb, C, Acta1, D, Myh7 in 34–35-week old WT, SKO and DKO mice. Values are related to WT mice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT, one-way ANOVA followed by Dunnetts comparison post-test, n = 5–8 per group. |
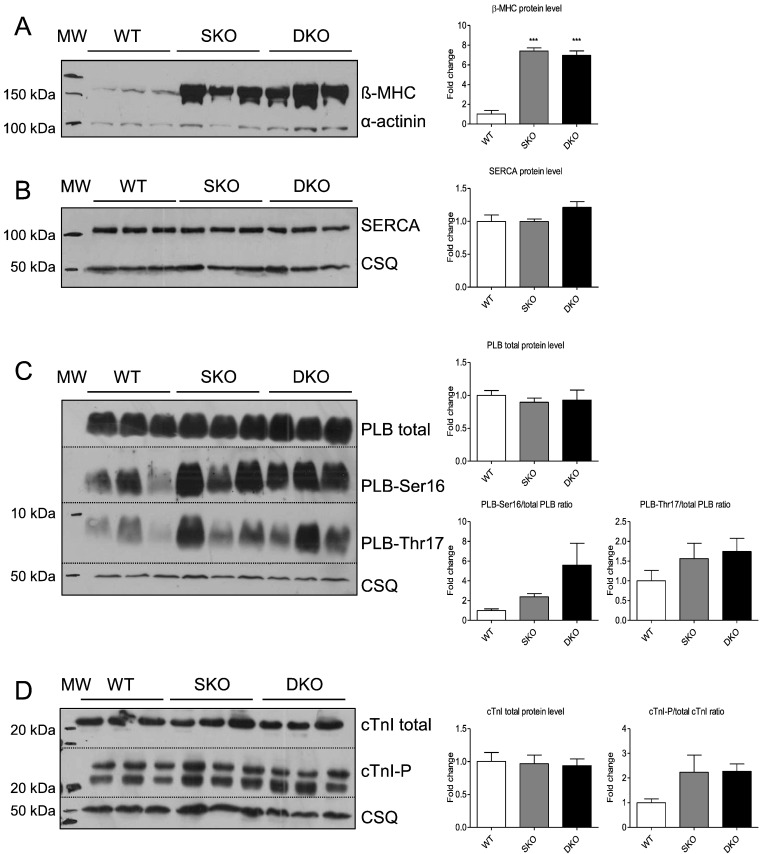
Both SKO and DKO showed higher beta-myosin heavy chain steady-state protein levels than WT (Fig. 4A). Quantification of sarcoplasmic reticulum Ca2 +-ATPase pump (SERCA) and total PLB protein levels revealed no differences between the three genotypes (Fig. 4B, C). PLB phosphorylation levels at Ser16 and Thr17 showed a stronger signal in SKO and DKO than in WT. Analysis of the total and phosphorylated states of the sarcomeric PKA target cardiac cTnI showed no difference in total levels, but a tendency to higher phosphorylation in SKO and DKO than in WT (Fig. 4D). This hyperphosphorylation could indicate an increased adrenergic signalling. I-1 mRNA levels were undetectable in DKO mice, but ~ 40% lower in SKO than in WT mice which is in agreement with lower I-1 mRNA and protein levels in failing human hearts and in response to chronic isoprenaline treatment [27] ; [28]. There was no difference in PP1 mRNA levels indicating no compensatory changes on gene expression level ( Supplemental Fig. 1).
|
|
|
Fig. 4. Analysis of calcium-handling proteins in WT, SKO and DKO mice. Representative Western blots stained with antibodies directed against A, β-myosin heavy chain (β-MHC), B, sarcoplasmic reticulum Ca2 +-ATPase pump (SERCA), C, total and phosphorylated phospholamban (PLB), and D, total and phosphorylated cardiac troponin I (cTnI) or alpha-actinin/calsequestrin (protein loading control). Molecular weight markers (MW) as indicated. Protein levels were normalized to alpha-actinin/calsequestrin and related to WT protein levels. Data are expressed as mean ± SEM, ***p < 0.001 vs. WT, one-way ANOVA followed by Dunnetts comparison post-test, n = 3–6. |
3.4. Long-term metoprolol treatment has no effect on cardiac function in Mybpc3 KI mice
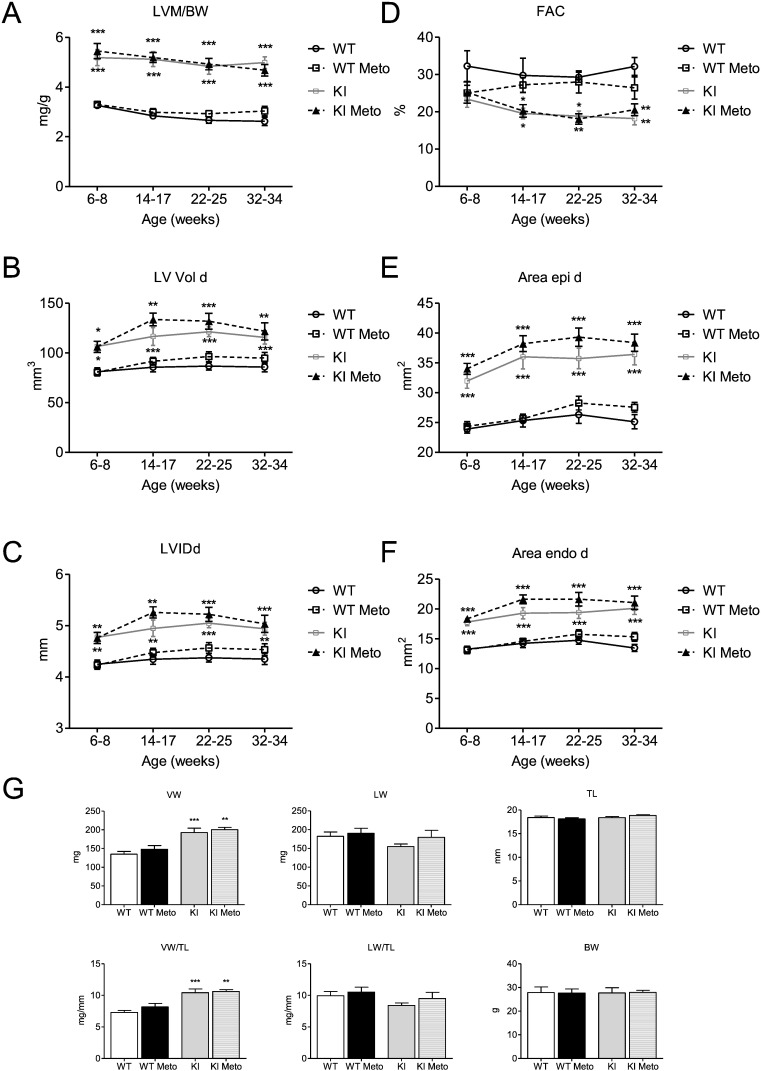
In order to evaluate whether a classical beta-AR blockade has a similarly detrimental effect on cardiac function as I-1 deficiency in the Mybpc3 KO mouse model, we supplemented the drinking water of Mybpc3 KI mice, another HCM mouse model, with the beta-AR antagonist metoprolol, starting at 6–8 weeks of age. Homozygous KI mice show only 10–20% of Mybpc3 protein and mRNA levels, and their disease phenotype mimics Mybpc3 KO and severe HCM cases [3]; [29] ; [30]. Cardiac function was measured by echocardiography before, during and at the end of the study. During the study none of the KI mice died. After 6 months of metoprolol treatment, no differences in LVM/BW, LV Vol d, LVIDd, FAC, Area epi and endo d were observed between treated and untreated KI mice (Fig. 5A–F). We also assessed ventricular, lung and body weight parameters at the end of the study. Similarly, no differences between treated and untreated KI mice could be seen (Fig. 5G).
|
|
|
Fig. 5. Longitudinal echocardiography study and body parameters of Mybpc3 WT and KI mice ± metoprolol. Heart function was measured in Mybpc3 WT and KI mice by echocardiography before (6–8 weeks of age) and during metoprolol treatment (14–17, 22–25, 32–34 weeks of age). A, Left ventricular mass/body weight ratio [LVM/BW], B, fractional area change [FAC], C, left ventricular enddiastolic volume [LV Vol d], D, left ventricular internal enddiastolic diameter [LVIDd], E and F, external (Area epi d) and internal (Area endo d) left ventricular enddiastolic areas were measured in Mybpc3 WT and KI mice ± metoprolol (Meto), n = 8–10. G, ventricular weight (VW), lung weight (LW), tibia length (TL), ventricular weight/tibia length ratio (VW/TL), lung weight/tibia length ratio (LW/TL) and body weight (BW) were assessed after 6 months of treatment, n = 8–10. Data are expressed as mean ± SEM, *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT, two-way ANOVA followed by Bonferroni comparison post-test or one-way ANOVA followed by Dunnetts comparison post-test. |
4. Discussion
Current treatment of human HCM is based on the use of beta-AR and calcium channel blockers. Both have negative inotropic effects and can reduce outflow tract obstruction. Moreover, they could be advantageous in light of the early concept of hyper-adrenergic state in HCM [7]; [8] ; [31]. Beta-AR blockers in particular have shown to be beneficial in patients with angina or dyspnea, particularly when associated with LVOT obstruction [9]. These effects are induced by lowering heart rate, contractility and stiffness of the ventricle, which results in improved ventricular relaxation, increased time for diastolic filling, and reduced tendency to arrhythmias [32] ; [33]. Despite these beneficial effects, the ultimate impact of beta-AR blockers on outcome in HCM patients remains undefined [34].
Experimental approaches in HCM mouse models have produced positive and negative results. Whereas treatment with drugs as diltiazem, losartan, spironolactone and HMG-CoA-reductase inhibitors has shown beneficial effects, application of calcineurin inhibitors worsened the phenotype [35]; [36]; [37]; [38] ; [39]. Lately, it was reported that genetic normalization of myofilament Ca2 + sensitivity by pseudophosphorylated TnI inhibits disease development in HCM tropomyosin mice [40]. Gene therapy approaches directed towards normalization of pathologically low mRNA and protein levels in Mybpc3 KI mice, either by repair of mutated RNA [41]; [42] ; [43] or by introduction of the correct full-length Mybpc3 cDNA have provided significant disease prevention [3].
This study shows the importance of a more comprehensive evaluation of a potential “therapeutic target/strategy” in different types of heart diseases to be really able to assess the therapeutic potential of such strategy. We tested the hypothesis that intracellular modulation of the beta-AR cascade by I-1 ablation could be advantageous in a Mybpc3 KO mouse model by reducing the sensitivity of beta-AR signalling without lowering heart rate or contractility (e.g. by lower LTCC and myofilament phosphorylation), but rather by targeting intracellular calcium handling. Similar to results we obtained in human heart failure samples and in rats after chronic ISO stimulation [27] ; [28], SKO mice demonstrated lower I-1 mRNA levels than WT mice, which could indicate a protective mechanism against excessive adrenergic drive in heart failure. We previously demonstrated that I-1 deficiency slightly reduced the inotropic response to beta-AR stimulation in isolated heart muscle preparations, but did not affect basal contractility [16]. Accordingly, in the current study we did not observe a difference in FAC between SKO and DKO mice. However, DKO exhibited even more pronounced LV dilatation and earlier death, clearly indicating that, in this Mybpc3 KO mouse model, I-1 deficiency has adverse effects albeit unchanged LV dimensions in single I-1 deficient mice [16]. There are two possible explanations. In heart failure, beta-AR blockers should not be initiated in acutely decompensated patients because of further worsening of pump function [44]. Both Mybpc3 mouse models which were used in the study show severe systolic and diastolic dysfunction and ventricular dilatation at the homozygous state. A high-dose beta-AR blocker treatment could therefore promote heart failure decompensation as seen in the case of the DKO. At first sight, the observation that beta-AR blockade by metoprolol did not worsen cardiac function in Mybpc3 KI does not support this theory. However, KI in this genetic background (Black Swiss) had a much better contractile function than the SKO, suggesting that this may well be due to a difference in severity of the cardiomyopathy phenotype in the two mouse models. And it was apparent that metoprolol treatment did not improve cardiac function or reduce the degree of hypertrophy in Mybpc3 KI mice. Thus, most likely, Mybpc3 KO mice with a severe form of cardiomyopathy need a certain level of adrenergic signalling downstream of the beta-AR receptor for compensation and this reserve was depressed by I-1 ablation.
An alternative or additional explanation for the adverse consequences of I-1 ablation in the DKO relates to diastolic function. Formerly we reported that SKO mice showed a relaxation deficit and higher PLB phosphorylation levels. In this study, SKO and DKO showed higher PLB and cTnI phosphorylation levels than the WT as well. This was unexpected for DKO mice since in a former study single I-1 KO mice presented PLB hypophosphorylation [16]. This higher phosphorylation status could be a compensational mechanism to accelerate Ca2 + uptake into the SR and therefore hasten relaxation [21]. In this respect, stimulation of beta-AR in Mybpc3-related HCM could be a beneficial compensatory mechanism that alleviates diastolic dysfunction caused by the relative or complete lack of Mybpc3. I-1 deficiency in the Mybpc3 KO model would then negatively interfere by misbalancing beta-adrenergic signalling and causing exaggerated cardiac hypertrophy and lower survival rates in DKO compared to SKO.
4.1. Study limitations
The study has several limitations. 1) While we used different approaches to understand the nature of the augmented phenotype in (I-1/Mybpc3 KO) mice, including gene and protein expression levels of genes usually deregulated in cardiomyopathy, we were not able to identify critical molecular difference between SKO and DKO mice with respect to the observed phenotype. An unbiased approach to identify potential genetic modifiers, e.g. by whole-genome transcriptomics or proteomics, was however beyond of scope of the current study. 2) Most importantly, Mybpc3 KO and KI mice do not exhibit a typical HCM phenotype. Whereas humans generally develop a HCM phenotype with only one allele mutated, heterozygous Mybpc3 KO and KI exhibit only mild signs of diastolic dysfunction, but no cardiac hypertrophy. The present experiments were therefore performed with homozygous KO/KI mice which are severely sick, but rather with a dilated cardiomyopathy phenotype and severe systolic and diastolic dysfunction. This mimics the rare cases of neonates with two diseased alleles [45] ; [46]. Both models are obviously not perfect for the more common form of heterozygous HCM with normal or even supra-normal ejection fraction. The results can therefore not be directly transferred to the human situation and further studies using heterozygous Mybpc3 KO and KI mouse models on an I-1 deficient background might help to elucidate the full picture of beta-AR signalling blockade in clinical settings of HCM. Yet, the clearly harmful effects of I-1 ablation in the Mybpc3 KO model indicate that beneficial effects seen previously under strong beta-AR stimulation [16] cannot be simply transferred to all cardiac pathologies. And, together with the lack of beneficial effect of beta-AR blocker treatment in the KI model, the data argue against a beneficial effect of beta-AR treatment on severe Mybpc3-related cardiomyopathies. 3) Finally we cannot rule out any adverse effects of I-1 ablation on extra cardiac tissue which might contribute to the observed phenotype in the DKO. Construction of a tissue-restricted I-1 KO mouse model may help in clarifying this issue in the future.
The following are the supplementary data related to this article.
Supplemental Fig. 1.
Gene expression of I-1 and PP1 in WT, SKO and DKO mice.
A, levels of mRNA of A, I-1 and B PP1 in 34–35-week-old WT, SKO and DKO mice.
Data are expressed as mean ± SEM, **p < 0.01 vs. WT, one-way ANOVA followed by Dunnetts comparison post-test, n = 5–8.
Supplementary tables
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG EL 270/5-1 and SFB 1002 to AEA) and the German Heart Foundation/German Foundation of Heart Research (F/28/12).
Disclosures
None.
Acknowledgment
We thank Marc N. Hirt and Giulia Mearini for discussion and Saskia Schlossarek for assistance in mouse mating and keeping.
References
- [1] P. Elliott, B. Andersson, E. Arbustini, Z. Bilinska, F. Cecchi, P. Charron, et al.; Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases; Eur Heart J, 29 (2) (Jan 2008), pp. 270–276 PubMed PMID: 17916581
- [2] F.W. Friedrich, L. Carrier; Genetics of hypertrophic and dilated cardiomyopathy; Curr Pharm Biotechnol, 13 (13) (Oct 2012), pp. 2467–2476 PubMed PMID: 22280421. Epub 2012/01/28.eng
- [3] G. Mearini, D. Stimpel, B. Geertz, F. Weinberger, E. Kramer, S. Schlossarek, et al.; Mybpc3 gene therapy for neonatal cardiomyopathy enables long-term disease prevention in mice; Nat Commun., 5 (2014), p. 5515 PubMed PMID: 25463264
- [4] D.C. Lefroy, R. de Silva, L. Choudhury, N.G. Uren, T. Crake, C.G. Rhodes, et al.; Diffuse reduction of myocardial beta-adrenoceptors in hypertrophic cardiomyopathy: a study with positron emission tomography; J Am Coll Cardiol, 22 (6) (Nov 15 1993), pp. 1653–1660 PubMed PMID: 8227834
- [5] S.T. Li, C.J. Tack, L. Fananapazir, D.S. Goldstein; Myocardial perfusion and sympathetic innervation in patients with hypertrophic cardiomyopathy; J Am Coll Cardiol, 35 (7) (Jun 2000), pp. 1867–1873 PubMed PMID: 10841237
- [6] P. Sipola, E. Vanninen, H.J. Aronen, K. Lauerma, S. Simula, P. Jaaskelainen, et al.; Cardiac adrenergic activity is associated with left ventricular hypertrophy in genetically homogeneous subjects with hypertrophic cardiomyopathy; J Nucl Med Allied Sci, 44 (4) (Apr 2003), pp. 487–493 PubMed PMID: 12679389
- [7] B.J. Gersh, B.J. Maron, R.O. Bonow, J.A. Dearani, M.A. Fifer, M.S. Link, et al.; ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons; J Am Coll Cardiol, 58 (25) (Dec 13 2011), pp. e212–e260 PubMed PMID: 22075469
- [8] Authors/Task Force m, P.M. Elliott, A. Anastasakis, M.A. Borger, M. Borggrefe, F. Cecchi, et al.; ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC); Eur Heart J, 35 (39) (Aug 29 2014), pp. 2733–2779 [PubMed PMID: 25173338]
- [9] R. Spoladore, M.S. Maron, R. D'Amato, P.G. Camici, I. Olivotto; Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence; Eur Heart J, 33 (14) (Jul 2012), pp. 1724–1733 [PubMed PMID: 22719025]
- [10] S. Herzig, J. Neumann; Effects of serine/threonine protein phosphatases on ion channels in excitable membranes; Physiol Rev, 80 (1) (Jan 2000), pp. 173–210 [PubMed PMID: 10617768]
- [11] C.J. Oliver, S. Shenolikar; Physiologic importance of protein phosphatase inhibitors; Front Biosci, 3 (Sep 1 1998), pp. D961–D972 [PubMed PMID: 9727084]
- [12] P. Nicolaou, R.J. Hajjar, E.G. Kranias; Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology; J Mol Cell Cardiol, 47 (3) (Sep 2009), pp. 365–371 [PubMed PMID: 19481088. Pubmed Central PMCID: 2716438]
- [13] A. El-Armouche, T. Rau, O. Zolk, D. Ditz, T. Pamminger, W.H. Zimmermann, et al.; Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes; FASEB J, 17 (3) (Mar 2003), pp. 437–439 [PubMed PMID: 12514122]
- [14] P. Nicolaou, P. Rodriguez, X. Ren, X. Zhou, J. Qian, S. Sadayappan, et al.; Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury; Circ Res, 104 (8) (Apr 24 2009), pp. 1012–1020 [PubMed PMID: 19299645. Pubmed Central PMCID: 2752882]
- [15] K. Ishikawa, K.M. Fish, L. Tilemann, K. Rapti, J. Aguero, C.G. Santos-Gallego, et al.; Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure; Mol Ther, 22 (12) (Dec 2014), pp. 2038–2045 [PubMed PMID: 25023328]
- [16] A. El-Armouche, K. Wittkopper, F. Degenhardt, F. Weinberger, M. Didie, I. Melnychenko, et al.; Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy; Cardiovasc Res, 80 (3) (Dec 1 2008), pp. 396–406 [PubMed PMID: 18689792. Epub 2008/08/12. Eng]
- [17] A. El-Armouche, T. Eschenhagen; Beta-adrenergic stimulation and myocardial function in the failing heart; Heart Fail Rev, 14 (4) (Dec 2009), pp. 225–241 [PubMed PMID: 19110970]
- [18] C.Y. Ho, P. Charron, P. Richard, F. Girolami, K.Y. Van Spaendonck-Zwarts, Y. Pinto; Genetic advances in sarcomeric cardiomyopathies: state of the art; Cardiovasc Res, 105 (4) (Apr 1 2015), pp. 397–408 PubMed PMID: 25634555. Pubmed Central PMCID: 4349164]
- [19] L. Carrier, R. Knoell, N. Vignier, D.I. Keller, P. Bausero, B. Prudhon, et al.; Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice; Cardiovasc Res, 63 (2) (Aug 1 2004), pp. 293–304 [PubMed PMID: 15249187]
- [20] O. Cazorla, S. Szilagyi, N. Vignier, G. Salazar, E. Kramer, G. Vassort, et al.; Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice; Cardiovasc Res, 69 (2) (Feb 1 2006), pp. 370–380 [PubMed PMID: 16380103]
- [21] L. Pohlmann, I. Kroger, N. Vignier, S. Schlossarek, E. Kramer, C. Coirault, et al.; Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes; Circ Res, 101 (9) (Oct 26 2007), pp. 928–938 [PubMed PMID: 17823372]
- [22] P.B. Allen, O. Hvalby, V. Jensen, M.L. Errington, M. Ramsay, F.A. Chaudhry, et al.; Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms; J Neurosci, 20 (10) (May 15 2000), pp. 3537–3543 [PubMed PMID: 10804194. Epub 2000/05/11. Eng]
- [23] B. Fraysse, F. Weinberger, S.C. Bardswell, F. Cuello, N. Vignier, B. Geertz, et al.; Increased myofilament Ca2 + sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice; J Mol Cell Cardiol, 52 (6) (Jun 2012), pp. 1299–1307 [PubMed PMID: 22465693. Pubmed Central PMCID: 3370652]
- [24] F.W. Friedrich, S. Reischmann, A. Schwalm, A. Unger, D. Ramanujam, J. Munch, et al.; FHL2 expression and variants in hypertrophic cardiomyopathy; Basic Res Cardiol, 109 (6) (Nov 2014), p. 451 [PubMed PMID: 25358972]
- [25] F.W. Friedrich, B.R. Wilding, S. Reischmann, C. Crocini, P. Lang, P. Charron, et al.; Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy; Hum Mol Genet, 21 (14) (Jul 15 2012), pp. 3237–3254 [PubMed PMID: 22523091. Epub 2012/04/24. Eng]
- [26] A. El-Armouche, L. Pohlmann, S. Schlossarek, J. Starbatty, Y.H. Yeh, S. Nattel, et al.; Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure; J Mol Cell Cardiol, 43 (2) (Aug 2007), pp. 223–229 [PubMed PMID: 17560599]
- [27] A. El-Armouche, F. Gocht, E. Jaeckel, K. Wittkopper, M. Peeck, T. Eschenhagen; Long-term beta-adrenergic stimulation leads to downregulation of protein phosphatase inhibitor-1 in the heart; Eur J Heart Fail, 9 (11) (Nov 2007), pp. 1077–1080 [PubMed PMID: 17921049]
- [28] A. El-Armouche, T. Pamminger, D. Ditz, O. Zolk, T. Eschenhagen; Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts; Cardiovasc Res, 61 (1) (Jan 1 2004), pp. 87–93 [PubMed PMID: 14732205]
- [29] S. Schlossarek, D.R. Englmann, K.R. Sultan, M. Sauer, T. Eschenhagen, L. Carrier; Defective proteolytic systems in Mybpc3-targeted mice with cardiac hypertrophy; Basic Res Cardiol, 107 (1) (Jan 2012), pp. 1–13 [PubMed PMID: 22189562. Epub 2011/12/23. Eng]
- [30] S. Schlossarek, F. Schuermann, B. Geertz, G. Mearini, T. Eschenhagen, L. Carrier; Adrenergic stress reveals septal hypertrophy and proteasome impairment in heterozygous Mybpc3-targeted knock-in mice; J Muscle Res Cell Motil, 33 (1) (May 2012), pp. 5–15 PubMed PMID: 22076249. Epub 2011/11/15. Eng]
- [31] B.J. Maron, W.J. McKenna, G.K. Danielson, L.J. Kappenberger, H.J. Kuhn, C.E. Seidman, et al.; American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines; Eur Heart J, 24 (21) (Nov 2003), pp. 1965–1991 PubMed PMID: 14585256
- [32] P. Spirito, C.E. Seidman, W.J. McKenna, B.J. Maron; The management of hypertrophic cardiomyopathy; N Engl J Med, 336 (11) (Mar 13 1997), pp. 775–785 PubMed PMID: 9052657
- [33] A.J. Marian; Contemporary treatment of hypertrophic cardiomyopathy; Tex Heart Inst J, 36 (3) (2009), pp. 194–204 PubMed PMID: 19568388. Pubmed Central PMCID: 2696493
- [34] B.J. Maron; Hypertrophic cardiomyopathy: a systematic review; JAMA, 287 (10) (2002), pp. 1308–1320 PubMed PMID: 11886323
- [35] D.S. Lim, S. Lutucuta, P. Bachireddy, K. Youker, A. Evans, M. Entman, et al.; Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy; Circulation, 103 (6) (Feb 13 2001), pp. 789–791 PubMed PMID: 11171784
- [36] A.J. Marian; Experimental therapies in hypertrophic cardiomyopathy; J Cardiovasc Transl Res, 2 (4) (Dec 2009), pp. 483–492 PubMed PMID: 20560006. Pubmed Central PMCID: 2904688. Epub 2010/06/19. eng
- [37] D. Fatkin, B.K. McConnell, J.O. Mudd, C. Semsarian, I.G. Moskowitz, F.J. Schoen, et al.; An abnormal Ca(2 +) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy; J Clin Invest, 106 (11) (Dec 2000), pp. 1351–1359 PubMed PMID: 11104788. Pubmed Central PMCID: 381468. Epub 2000/12/06. eng
- [38] C. Semsarian, I. Ahmad, M. Giewat, D. Georgakopoulos, J.P. Schmitt, B.K. McConnell, et al.; The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model; J Clin Invest, 109 (8) (2002), pp. 1013–1020 PubMed PMID: 11956238
- [39] N. Tsybouleva, L. Zhang, S. Chen, R. Patel, S. Lutucuta, S. Nemoto, et al.; Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy; Circulation, 109 (10) (Mar 16 2004), pp. 1284–1291 PubMed PMID: 14993121
- [40] M.L. Alves, F.A. Dias, R.D. Gaffin, J.N. Simon, E.M. Montminy, B.J. Biesiadecki, et al.; Desensitization of myofilaments to Ca2 + as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins; Circ Cardiovasc Genet, 7 (2) (Apr 1 2014), pp. 132–143 PubMed PMID: 24585742
- [41] V. Behrens-Gawlik, G. Mearini, C. Gedicke-Hornung, P. Richard, L. Carrier; MYBPC3 in hypertrophic cardiomyopathy: from mutation identification to RNA-based correction; Pflugers Arch, 466 (2) (Feb 2014), pp. 215–223 PubMed PMID: 24337823. Epub 2013/12/18. eng
- [42] C. Gedicke-Hornung, V. Behrens-Gawlik, S. Reischmann, B. Geertz, D. Stimpel, F. Weinberger, et al.; Rescue of cardiomyopathy through U7snRNA-mediated exon skipping in Mybpc3-targeted knock-in mice; EMBO Mol Med, 5 (7) (Jul 2013), pp. 1128–1145 PubMed PMID: 23716398. Pubmed Central PMCID: 3721478. Epub 2013/05/30. eng
- [43] G. Mearini, D. Stimpel, E. Kramer, B. Geertz, I. Braren, C. Gedicke-Hornung, et al.; Repair of Mybpc3 mRNA by 5′-trans-splicing in a mouse model of hypertrophic cardiomyopathy; Mol Ther Nucleic Acids, 2 (2013) e102. PubMed PMID: 23820890. Epub 2013/07/04. eng
- [44] J. McMurray, A. Cohen-Solal, R. Dietz, E. Eichhorn, L. Erhardt, F.D. Hobbs, et al.; Practical recommendations for the use of ACE inhibitors, beta-blockers, aldosterone antagonists and angiotensin receptor blockers in heart failure: putting guidelines into practice; Eur J Heart Fail, 7 (5) (Aug 2005), pp. 710–721 PubMed PMID: 16087129
- [45] S.A. El-Saiedi, Z.S. Seliem, R.I. Esmail; Hypertrophic cardiomyopathy: prognostic factors and survival analysis in 128 Egyptian patients; Cardiol Young, 24 (4) (Aug 2014), pp. 702–708 PubMed PMID: 23895893
- [46] B. Xin, E. Puffenberger, J. Tumbush, J.R. Bockoven, H. Wang; Homozygosity for a novel splice site mutation in the cardiac myosin-binding protein C gene causes severe neonatal hypertrophic cardiomyopathy; Am J Med Genet A, 143A (22) (Nov 15 2007), pp. 2662–2667 PubMed PMID: 17937428
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?