m (Scipediacontent moved page Draft Content 999917461 to Walker-Sperling et al 2016a) |
|||
| Line 404: | Line 404: | ||
<span id='ec0005'></span> | <span id='ec0005'></span> | ||
| − | [[Image:draft_Content_999917461-mmc_pdf.gif|center|link=Walker- | + | [[Image:draft_Content_999917461-mmc_pdf.gif|center|link=Media:Walker-Sperling_et_al_2016a_4012_mmc1.pdf]] |
<span style="text-align: center; font-size: 75%;"> | <span style="text-align: center; font-size: 75%;"> | ||
Latest revision as of 12:06, 14 November 2017
Highlights
- Latency reversal agents can reactivate HIV-1 expression in latently infected cells.
- CD8 T cells from HIV-1 infected patients did not eliminate reactivated latently infected cells.
- This finding can partially be explained by our data showing that latency reversal agents affect the function of CD8 + T cells.
Latently infected CD4 + T cells are a major barrier to the cure of HIV-1 infection. One strategy of eliminating these cells involves inducing viral transcription with small molecules (latency reversal agents or LRAs) which would result in the recognition of these cells by the immune system. We show here that CD8 + T cells were not able to eliminate CD4 + T cells from HIV-1-infected patients following stimulation with LRAs. Our data suggests that this may be partially because some LRAs affect the function of CD8 + T cells. Thus it will be critical to select LRAs that do not cause immune suppression.
Abstract
Shock and kill strategies involving the use of small molecules to induce viral transcription in resting CD4 + T cells (shock) followed by immune mediated clearance of the reactivated cells (kill), have been proposed as a method of eliminating latently infected CD4 + T cells. The combination of the histone deacetylase (HDAC) inhibitor romidepsin and protein kinase C (PKC) agonist bryostatin-1 is very effective at reversing latency in vitro. However, we found that primary HIV-1 specific CD8 + T cells were not able to eliminate autologous resting CD4 + T cells that had been reactivated with these drugs. We tested the hypothesis that the drugs affected primary CD8 + T cell function and found that both agents had inhibitory effects on the suppressive capacity of HIV-specific CD8 + T cells from patients who control viral replication without antiretroviral therapy (elite suppressors/controllers). The inhibitory effect was additive and multi-factorial in nature. These inhibitory effects were not seen with prostratin, another PKC agonist, either alone or in combination with JQ1, a bromodomain-containing protein 4 inhibitor. Our results suggest that because of their adverse effects on primary CD8 + T cells, some LRAs may cause immune-suppression and therefore should be used with caution in shock and kill strategies.
Keywords
HIV-1 ; Eradication ; Latency reversal agents ; Elite suppressors ; Elite controllers ; CD8 + T cells
1. Introduction
Latently infected CD4 + T cells are the major barrier to HIV-1 cure efforts. The cells contain integrated proviruses that are transcriptionally silent and thus able to evade detection and clearance by the immune system. The shock-and-kill cure strategy seeks to first reactivate these latent viruses without causing global T cell activation followed by clearance of the reactivated cells by the immune system (reviewed in Siliciano and Siliciano, 2013 and Archin and Margolis, 2014 ). Latency reactivating agents (LRAs) are drugs that induce HIV-1 transcription. Notable drug classes include PKC agonists and HDAC inhibitors (HDACi), which have been very effective in inducing HIV-1 transcription in cell lines (Contreras et al., 2009 , Xing et al., 2011 , Li et al., 2013 and DeChristopher et al., 2012 ). Unfortunately, in vitro experiments with primary resting CD4 T cells from patients on suppressive antiretroviral therapy (ART) regimens suggest that most individual LRAs are unable to induce substantive amounts of HIV-1 transcription with the notable exception of PKC agonists bryostatin-1-1 (Bullen et al., 2014 ) and ingenol (Spivak et al., 2015 ). However, LRA combinations in the same system are capable of inducing significant HIV-1 transcription (Laird et al., 2015 , Jiang et al., 2015 and Darcis et al., 2015 ).
The other half of the cure strategy deals with killing newly reactivated infected CD4 + T cells. Recent experiments suggest that reactivation from latency is not enough to induce cell death (Shan et al., 2012 ), and therefore there may be a need for immune mediated eradication. Expanded CD8 + T cell lines were able to clear reactivated latently infected resting CD4 + T cells following exposure to the HDAC inhibitor, vorinostat (Sung et al., 2015 ). However primary CD8 + T cells from patients on suppressive ART regimens that were pre-stimulated with overlapping Gag peptides were unable to consistently reduce the amount of HIV-1 mRNA induced from autologous resting CD4 + T cells that were activated with PMA and ionomycin (Walker-Sperling et al., 2015 ).
The combination of romidepsin and bryostatin-1 has been shown to be one of the best inducers of latent HIV-1 in primary CD4 + T cells (Laird et al., 2015 ). However, bryostatin-1 has been showed to be involved in the modulation of NFkB and NFAT (Williams et al., 2004 ) and romidepsin is known to affect the function of NK cells and CD8 + T cells (Kelly-Sell et al., 2012 and Jones et al., 2014 ). Other HDAC inhibitors have furthermore been known to induce Treg cells in vitro (Akimova et al., 2010 and Tao et al., 2007 ). The immunomodulatory activity of the two drug classes thought to be most promising in cure efforts therefore needs to be further studied in the context of CD8 + T cell elimination of reactivated latently infected CD4 + T cells.
In this study, we sought to determine the ability of HIV-specific CD8 + T cells from patients with progressive HIV-1 disease on ART (chronic progressors) to kill HIV-infected CD4 + T cells after treatment with LRAs. To elucidate the contribution of the drug treatments the HIV-specific response, suppression of infection was examined with elite suppressor CD8 + T cells that had been pre-treated with different LRAs, including an HDAC inhibitor, a bromodomain-containing protein 4 inhibitor, and multiple PKC agonists. Finally, we examined the mechanisms that may have contributed to the effects of drug treatment on CD8 + T cell function. Our results have implications for the HIV-1 cure agenda.
2. Methods
2.1. Donor blood samples
HIV-1 positive and HIV-1 negative blood samples were obtained from donors with written, informed consent and handled according to a Johns Hopkins University IRB approved protocol. The chronic progressors studied were HIV-1 positive individuals who were started on suppressive ART therapy during chronic infection and have a viral load of < 20 copies of HIV RNA/mL. Elite suppressors are patients who have maintained undetectable viral loads without antiretroviral therapy. The clinical characteristics of the patients are summarized in Table 1 .
| Subject | Current CD4 + T cell count | Nadir CD4 + T cell count | Time on suppressive regimen | Current regiment | HLA-A | HLA-B |
|---|---|---|---|---|---|---|
| CP8 | 424 | 18 | 8 years | 3TC, RAL EFV | 1, 68 | 57, 58 |
| CP9 | 991 | 190 | 8 years | TDF, FTC, DRV/c | 34, 68 | 58, 81 |
| CP11 | 1032 | 177 | 8 years | TDF, FTC, DRV/r | 2, 11 | 25, 57 |
| CP 14 | 646 | 12 | 4 years | DRV/r, DTG | ||
| CP 16 | 921 | 203 | 5 years | 3TC, ABC, DTG | 29, 20 | 42, 81 |
| CP25 | 584 | NA | 4 years | TDF, FTC, RAL | 3, 30 | 8, 42 |
| ES 3 | 1149 | NA | NA | NA | 25, 68 | 51, 57 |
| ES 6 | 601 | NA | NA | NA | 23 | 15, 57 |
| ES 9 | 798 | NA | NA | NA | 2, 30 | 27, 57 |
| ES 22 | 1033 | NA | NA | NA | 30, 31 | 15, 57 |
| ES 24 | 1742 | NA | NA | NA | 24, 30 | 7, 57 |
| ES 31 | 1236 | NA | NA | NA | 3 | 27, 58 |
3TC: lamivudine, ABC: abacavir, FTC: emtricitabine, TDF: tenofovir, DTG: dolutegravir, EFV: efavirenz, RAL: raltegravir, DRV/c: cobicistat boosted darunavir, DRV/r: ritonavir boosted darunavir.
NA: Not applicable.
2.2. Primary cell isolations
PBMCs were obtained from whole blood via Ficoll-Paque PLUS gradient centrifugation (GE Healthcare Life Sciences). PBMCs underwent negative selection for CD4 + T cells using the MACS system (CD4 Isolation Kit, Miltenyi Biotech). Resting CD4 + T cells were further isolated from the bulk population by depleting CD25 +, CD69 +, and HLA-DR + cells (CD25 microbeads, CD69 Isolation Kit, and HLA-DR microbeads; Miltenyi Biotech). When applicable, CD8 + T cells were obtained via positive selection from PBMCs (CD8 microbeads, Miltenyi Biotech) prior to any negative selection performed in experiments described below.
2.3. Latency reactivation ex vivo and autologous suppression
Resting CD4 + T cells isolated from fresh blood samples from ART-suppressed individuals as described above (“Primary Cell Isolation”) were plated in 12-well plates with 5 × 106 (DeChristopher et al., 2012 ) cells per replicate in non-stimulating media (RPMI 1640 + Glutamax, 10% FBS) and treated alone for six hours with the combination of bryostatin-1 (B, 10 nM; Sigma Aldrich) and romidepsin (R, 40 nM; Selleck Chemicals) in the presence of efavirenz (EFV, 10 μM) and raltegravir (RAL, 4 μM) to prevent new infection and more closely mimic in vivo conditions. CD8 + T cells were isolated at this time as described above from PBMCs that had been previously stimulated for seven days in the presence of 100 U IL-2/mL and overlapping consensus Gag and Nef peptides (10 μg/mL; AIDS Reagent Database). At the conclusion of the six hours, the pre-stimulated CD8 + T cells were co-cultured with the resting CD4 + T cells at a 1:1 effector:target ratio and concentration of 5 × 106 (DeChristopher et al., 2012 ) cells/mL for another 18 h in the presence of B/R, EFV, and RAL at the same concentration as the initial treatment. The CD8 + T cells were not washed prior to co-culture with resting CD4 + T cells. In a second set of experiments, the CD8 + and CD4 + T cells were co-cultured together for 24 h in the presence of B/R, EFV, and RAL. For each replicate, at the conclusion of the full 24 h, supernatant samples and the full 5 × 106 (DeChristopher et al., 2012 ) cell samples were harvested and placed in TRIzol LS and TRIzol (Life Technologies), respectively, for the isolation of supernatant and cell-associated RNA.
2.4. Isolation and quantification of cell-associated and supernatant HIV-1 mRNA
Cell-associated and supernatant RNA were isolated, and the HIV-1 mRNA present in those samples was then quantified as previously described (Bullen et al., 2014 , Laird et al., 2015 and Walker-Sperling et al., 2015 ). Supernatant RNA samples were measured on a Roche LightCycler 480 Real-Time PCR thermocycler with TaqMan Fast Advanced Mastermix (Applied Biosystems) run as per manufacturers instructions and the following primers: Forward (5′ → 3′) CAGATGCTGCATATAAGCAGCTG (9501–9523), Reverse (5′ → 3′) TTTTTTTTTTTTTTTTTTTTTTTTGAAGCAC (9629-poly A). The probe used is as follows: (5′ → 3′) FAM-CCTGTACTGGGTCTCTCTGG-MGB (9531–9550) (all nucleotide coordinates relative to HXB2 consensus sequence). The molecular standard curves used for the quantification were generated using serial dilutions of a TOPO plasmid containing the final 352 nucleotides of the HIV-1 genomic RNA with the addition of 30 deoxyadenosines on the 5′ end to mimic the poly-A tail.
2.5. Latency reactivation agents' effects on autologous suppression of ex vivo infection
Autologous bulk CD8 + and CD4 + T cells were freshly isolated from PBMCs from elite suppressors as described above (“Primary Cell Isolation”) for a modified version of a previously described HIV suppression assay (Buckheit et al., 2012 ). CD8 + T cells were treated for six hours in non-stimulating media (RPMI 1640 + Glutamax, 10% FBS) with either nothing, DMSO, romidepsin (40 nM), JQ1 (1 μM; Sigma Aldrich), vorinostat (335 nM), panobinostat (30 nM), bryostatin-1 at three concentrations (10 nM, 1 nM, 0.1 nM), or prostratin at two concentrations (1 μM, 0.3 μM; Sigma Aldrich) alone or in the combinations of romidepsin and bryostatin-1, romidepsin and prostratin, or bryostatin-1 and JQ1 at those concentrations. Meanwhile, the bulk CD4 + T cells were spinoculated at 1200 × g for two hours at 37 °C with HIV-1NL4 − 3 ∆ Env − GFP , a replication incompetent lab strain pseudovirus with env replaced with gfp and whose expression is controlled by the HIV promoter. At the conclusion of the six-hour drug treatments, the drug was washed from the CD8 + T cells before the cells were added in a 1:1 effector:target ratio to the spinoculated CD4 + T cells. The cells co-cultured in nonstimulating media (RPMI 1640 + Glutamax, 10% FBS) were incubated for three days prior to FACS analysis.
2.6. Bryostatin-1 treatment and its effects on cytokine production
PBMCs isolated from elite suppressors were plated at a concentration of 1 × 106 (DeChristopher et al., 2012 ) cells per mL in 48-well plates and treated for six hours with nothing, DMSO, romidepsin (40 nM), bryostatin-1 (10 nM), and the combination of romidepsin and bryostatin-1. After treatment, cells were washed and then re-plated as before in non-stimulating media (RPMI 1640 + Glutamax, 10% FBS). All samples were cultured with Golgi Plug and Golgi Stop (BD Biosciences) as per manufacturers instructions and 1 μg/mL of anti-CD28 and anti-CD49d antibodies (NA/LE anti-CD28 clone CD28.2, anti-CD49d clone 9F10; BD Biosciences). The no-stimulation control had no additional treatment added, and the two stimulation conditions were incubated with 10 μg/mL overlapping consensus Gag peptides and 1 μg/mL anti-CD3 for stimulation (NA/LE anti-CD3 clone HIT3a; BD Biosciences), respectively.
2.7. General effects of latency reactivation agents on immune markers and cell death
PBMCs isolated from HIV-negative donor blood were plated at a concentration of 1 × 106 (DeChristopher et al., 2012 ) cells per mL in 48-well plates and treated for six hours with nothing, DMSO, romidepsin (40 nM), bryostatin-1 at three concentrations (10 nM, 1 nM, 0.1 nM), prostratin (0.3 μM), and the combination of romidepsin (40 nM) and bryostatin-1 (10 nM). The doses of these drugs were selected based on the concentrations needed to reverse latency either alone or in combination (Laird et al., 2015 ). 40 nM of romidepsin is below the concentration of the plasma levels achieved in patients treated with this drug for lymphoma (Wei et al., 2014 ). Plasma bryostatin-1 levels of close to 1 nM have been achieved in patients receiving the highest tolerated dose of the drug (Smith et al., 2011 ). Two sets of cultures were set aside for analysis by FACS at six hours post-treatment and 18 hours post-treatment. For the rest of the cultures, cells were washed after six hours of drug treatment prior to replating in fresh plates at the same concentration of cells in non-stimulating media (RPMI 1640 + Glutamax, 10% FBS) either in the presence or absence of 1 μg/mL anti-CD3/CD28 antibodies (NA/LE anti-CD3 clone HIT3a, anti-CD28 clone CD28.2; BD Biosciences) for an additional 1, 2, or 3 days before FACS analysis.
2.8. FACS analysis of suppression and immune markers
For the suppression experiments, samples were analyzed for infected CD4 + T cells by staining for CD3 (PacBlue, BD Biosciences), CD4 (BV605, Biolegend), and CD8 (APC-H7, BD Biosciences) and examining for the GFP + (pseudovirus infected) cells. The amount of suppression was calculated by comparing the amount of infected CD4 + T cells with CD8 + T cell co-culture to those without effector cell co-culture (% Suppression = [1 − (% GFP + CD4 + T cells cultured with CD8 + T cells) / (% GFP + CD4 + T cells without effectors)] × 100%). Intracellular cytokine expression was determined with the following panel: CD3·PacBlue, CD4·BV605, CD8·APC-H7, CD69·APC (Biolegend), IL-2·PE (BD Biosciences), TNFα·PE-Cy7 (BD Biosciences), IFNγ·PerCP-Cy5.5 (BD Biosciences), and Perforin-FITC (Cell Sciences). Immune markers and cell death were examined in the HIV-negative donors' cells via three staining panels (Panel A: CD3·APC-Cy7 [Biolegend], CD4·BV605, CD8·APC [BD Biosciences], PD-1·FITC [Biolegend]; Panel B: CD3·PE [BD Biosciences], CD4·BV605, CD8·APC-H7, CD69·APC, 7-AAD [BD Biosciences], Annexin V·V450 [BD Biosciences]; Panel C: CD3·PacBlue, CD8·APC-H7, CD69·BV605, CD160·PE [Biolegend], TIM-3·PE-Cy7 [Biolegend], 2B4·APC [BD Biosciences]). CD69, the exhaustion markers, and Annexin V expression are shown as raw data, but expression of CD3 is compared via MFI ratio (MFI ratio = [MFI of marker in treatment]/[MFI of marker in no treatment]). All samples were run on a BD FACSCantoII flow cytometer and analyzed in FlowJo vX.0.7.
2.9. Statistics
Statistical analyses performed for HIV-1 RNA (Fig. 1 ) were conducted using Wilcoxon matched-pair signed-rank test, the nonparametric alternative to the paired t -test as previously described ( Walker-Sperling et al., 2015 ). Descriptive statistics for other experiments are presented as means and standard deviations. Comparisons of treatment groups to the control (NT) were conducted using repeated measures ANOVA model with adjusted pair-wise comparisons to NT via Dunnetts correction. Strength of evidence, threshold p -values, will be presented as: ns (> 0.05), * (< 0.05), ** (< 0.01), and *** (< 0.001). Parametric methods were used due to the failure of nonparametric to detect significance for paired data with sample size less than six. All statistics and graphics were performed with GraphPad Prism 6.
|
|
|
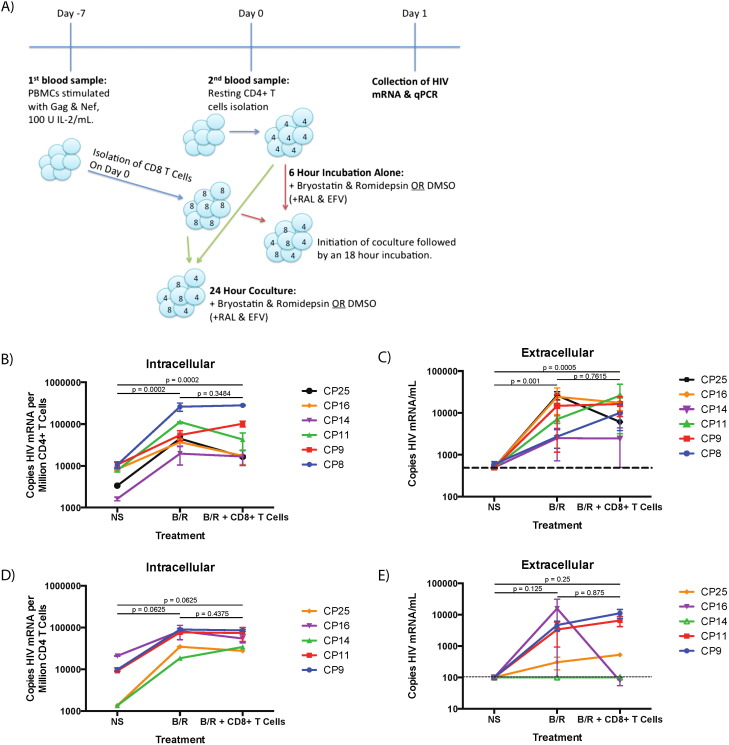
Fig. 1. Nef- and Gag-stimulated CD8 + T cells from chronic progressors are not capable of eliminating newly-reactivated autologous CD4 + T cells following bryostatin-1/romidepsin-treatment. A) Schematic of methods. Blue arrows indicate methods common to both experiments, red arrows indicate methods for Fig. 1 B and C, and green arrows indicate methods for Fig. 1 D and E. B) Level of cell-associated HIV-1 mRNA seen with no stimulation (NS), treatment with bryostatin-1/romidepsin (B/R) or treatment with B/R and co-culture with CD8 + T cells for 18 h. No significance is indicated by n.s., and the listed p -value indicates the level of significance as determined by a Wilcoxon signed rank test for significance. C) Level of HIV-1 mRNA present in culture supernatant for 6-hour drug treatment followed by 18-hour CD8 + T cell co-culture. Dotted line indicates the level of detection (500 copies HIV mRNA/mL). The listed p -value indicates the level of significance as determined by a Wilcoxon signed rank test for significance. D) Cell-associated HIV mRNA from 24-hour drug treatment and CD8 + T cell co-culture with resting CD4 + T cells. The listed p -value indicates the level of significance as determined by a Wilcoxon signed rank test for significance. E) Supernatant HIV mRNA from 24-hour drug treatment and CD8 + T cell co-culture with resting CD4 + T cells. The listed p -value indicates the level of significance as determined by a Wilcoxon signed rank test for significance. Dotted line indicates the level of detection (100 copies HIV mRNA/mL). Mean ± standard error of 1–3 replicates is shown for each individual. |
3. Results
3.1. Ex vivo reactivation of latently infected CD4 + T Cells and CD8 + effector-mediated elimination
Resting CD4 + T cells from six ART-suppressed HIV-1 + individuals were treated with 10 nM bryostatin-1 and 40 nM romidepsin (B/R) for twenty-four hours to reactivate latent HIV-1 proviruses. A subset of samples from each of the individuals was further co-cultured with autologous CD8 + T cells that had been previously cultured for 7 days in the presence of IL-2 and overlapping consensus Gag and Nef peptides to determine whether these immune effectors cells could eliminate latently infected cells. In a prior study we demonstrated that viral release occurred as early as 6 h after resting CD4 + T cell stimulation (Walker-Sperling et al., 2015 ), so the CD8 + T cells were added at 6 h to minimize their exposure to the latency reversal agents. In all six individuals, cell-associated HIV mRNA was increased by a median of 12.72-fold due to B/R treatment as compared to resting CD4 + T cells cultured in the absence of drugs (p < 0.0002, Fig. 1 B). However, the co-culture of B/R-treated resting CD4 + T cells with stimulated CD8 + T cells did not result in a significant decrease in the amount of HIV-1 mRNA, although three of the six individuals showed a trend towards a decrease in the amount of cell-associated HIV-1 mRNA (Fig. 1 B). The effect of drugs and CD8 + T cells on the release of virus into culture supernatant was also examined. HIV-1 mRNA present in culture supernatant increased significantly from an undetectable baseline (500 copies/mL) following B/R treatment to a median of 5281.6 copies/ml (p < 0.001), and there was no significant difference seen when the B/R-treated CD4 + T cells were co-cultured with CD8 + T cells (Fig. 1 C). Of the six individuals, only CP25 had a decrease in both cell-associated and supernatant HIV-1 mRNA due to co-culture of B/R-treated resting CD4 + T cells with CD8 + T cells (Fig. 1 C). We repeated the experiments with CD8 + T cells present at the time point 0 to ensure that the failure to eliminate reactivated CD4 + T cells was not due to early transcription and translation in the absence of the effector cells. However, there was still no significant decrease in either intracellular (Fig. 1 D) or extracellular mRNA expression (Fig. 1 E) when the stimulated CD4 + T cells were co-cultured with CD8 + T cells.
3.2. Elite suppressor CD8 + T cell responses after ex vivo treatment with latency reactivating agents
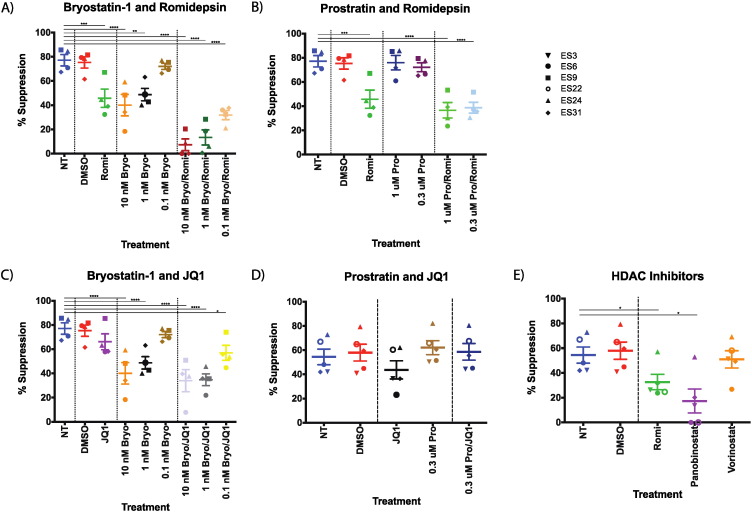
To elucidate whether or not the lack of an effective response to reactivated HIV-1 from latently infected CD4 + T cells in the chronic progressors was due to host factors or the drugs themselves we studied the effects of the drugs on HIV-specific CD8 + T cell responses. Elite suppressors were used for these studies, as they are known to have qualitatively superior HIV-specific CD8 + T cell responses as compared to the average chronic progressor (Betts et al., 2006 , Migueles et al., 2002 , Sáez-Cirión et al., 2007 , Migueles et al., 2008 and Hersperger et al., 2010 ). CD4 + T cells from ES were infected with replication incompetent HIV-1NL4 − 3 ∆ Env − GFP pseudovirus and co-cultured with autologous CD8 + T cells that had been previously incubated with a variety of latency reversing agents (LRAs), as previously described (Buckheit et al., 2012 and Pohlmeyer et al., 2013 ). DMSO, the vehicle for all the drugs, had no effect on the CD8 + T cell-mediated suppression as compared to no treatment (Fig. 2 A–E). Romidepsin (40 nM throughout) alone and bryostatin-1 at either 10 nM or 1 nM significantly inhibited the ability of elite suppressor CD8 T cells to suppress infection as compared to untreated CD8 + T cells (Fig. 2 A). The combination of romidepsin and all three concentrations of bryostatin-1 tested (10 nM and 1 nM significantly inhibited suppression as compared to untreated CD8 + T cells, and the combinations of romidepsin and bryostatin-1 at either 10 nM or 1 nM were much more inhibitory than either drug alone, with an 81.8% and 84.0% reduction in the amount of suppression seen for the combination of bryostatin at 10 nM and romidepsin from each drug alone, respectively, and 72.6% and 70.8% reduction for the combination of bryostatin at 10 nM and romidepsin from each alone.
|
|
|
Fig. 2. Elite suppressor CD8 + T cell responses are inhibited by bryostatin-1 and romidepsin alone and in combination. CD8 + T cells from 4 elite suppressors were preincubated with the indicated LRAs for six hours prior to the addition to autologous CD4 + T cells infected with lab strain HIV-1 pseudovirus in a 1:1 effector:target ratio and the percent suppression of viral replication was determined. Triplicates were performed and the mean values are shown for each individual. For panels D and E, data from 2 separate experiments with cells from ES6, ES22, and ES24 were averaged, and data from an additional elite suppressor was included. A) Comparison of bryostatin-1 and romidepsin treatments. B) Comparison of prostratin and romidepsin treatments. C) Comparison of bryostatin-1 and JQ1 treatments. D) Comparison of prostratin and JQ1 treatment. E) Comparison of romidepsin and other HDAC inhibitors. One-way repeated measures ANOVAs were used to determine significance for each of the two sets of experiments. Symbols directly above treatments indicate differences from NT, no treatment. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. |
In order to determine whether the inhibition of suppression is unique to bryostatin-1 or is a feature of PKC agonists, CD8 + T cells from elite suppressors were also treated with prostratin at either 1 μM or 0.3 μM alone or in conjunction with romidepsin. As opposed to bryostatin-1, prostratin alone at either concentration had no significant effect upon the suppressive capacity of the CD8 + T cells (Fig. 2 B). The combination of prostratin at 1 μM and 0.3 μM with romidepsin significantly inhibited the suppression of infection (p < 0.0001), but this inhibition was very similar to the inhibition seen by romidepsin alone (Fig. 2 B).
The effect of JQ1, a bromodomain-containing protein 4 inhibitor, was also examined alone and in conjunction with bryostatin-1 or prostratin as these combinations of drugs have also been shown to be effective at reversing latency in vitro (Laird et al., 2015 ). Treatment of CD8 + T cells with JQ1 (1 μM) alone resulted in a slight, nonsignificant decrease in CD8 + T cell suppression (Fig. 2 C). A significant decrease in CD8 + T cell-mediated suppression was seen when JQ1 was given in combination with bryostatin-1 at 10 nM and 1 nM (p < 0.001 for both), but this was not significantly different from the inhibition seen with bryostatin-1 alone at either concentration (Fig. 2 C). In contrast, the combination of prostratin and JQ-1 did not have a significant effect on the CD8 + T cell function (Fig. 2 D).
In order to determine whether romidepsins effect on CD8 + T cell suppressive activity was unique to this drug or a feature of all HDAC inhibitors, we compared the effects of romidepsin vorionostat and panobinostat on ES CD8 + T cell function. The concentrations used were based on concentrations that had been shown to be effective in prior studies (Archin et al., 2012 and Laird et al., 2015 ) and were similar to levels that have been achieved in vivo (Wei et al., 2014 , Rubin et al., 2006 and Archin et al., 2012 , Bauer et al., 2014 ). While romidepsin and panobinostat had significant inhibitory effects on ES CD8 + T cells, vorinostat did not cause significant suppression (Fig. 2 E). These results are similar to results obtained with HIV-specific CD8 + T cell clones (Jones et al., 2014 ).
3.3. CD8 + T cell cytokine production after LRA treatment
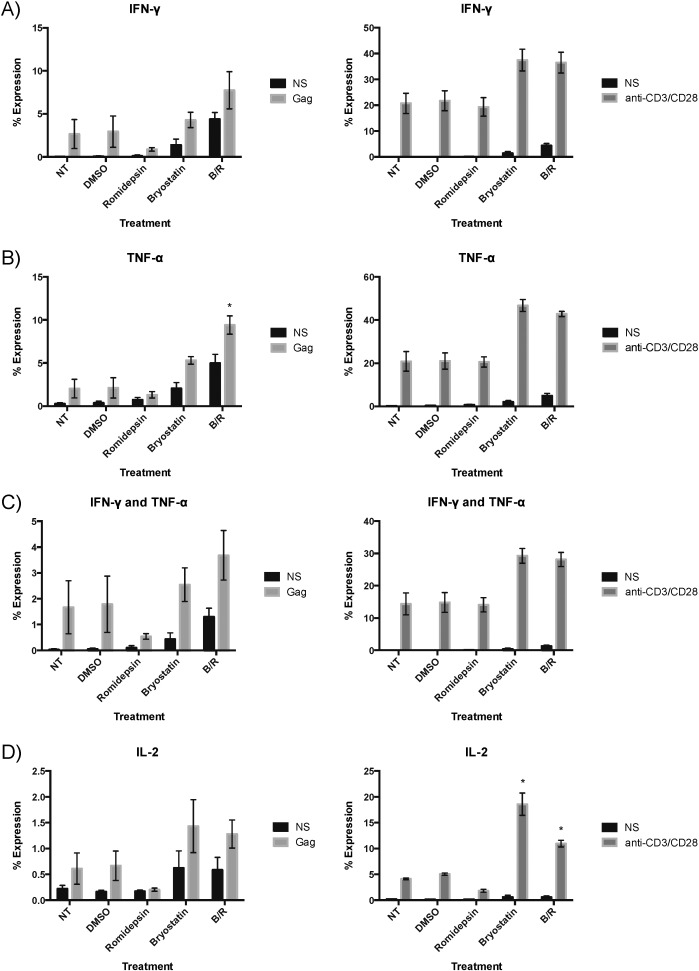
We next sought to find a mechanism for the inhibition of suppression due to bryostatin-1 treatment and first examined the ability of elite suppressor HIV-specific CD8 + T cells to produce cytokines after a 6-hour treatment with bryostatin-1 and romidepsin followed by 12-h of stimulation with either Gag peptides or anti-CD3 and CD28 monoclonal antibodies. Bryostatin-1 treatment alone tended to cause an increase in the percentage of cells that produced TNF-α, both TNF-α and IFN-γ, and IL-2 following anti-CD3/CD28 stimulation (for IL-2, p < 0.05; Fig. 3 B). In contrast, the B/R combination caused an increase in the percentage of cytokine-producing CD8 + T cells at baseline (IFN-γ, TNF-α, and IFN-γ and TNF-α), and following stimulation with Gag peptides (TNF-α; p < 0.05) and anti-CD3/CD28 monoclonal antibodies (TNF-α, TNF-α and IFN-γ, and IL-2; for IL-2 only, p < 0.05). Thus it appears that a decrease in cytokine expression was not the mechanism of suppression of CD8 + T cell antiviral activity.
|
|
|
Fig. 3. Production of IFN-γ, TNF-α, and IL-2 by CD8 + T cells increases with bryostatin-1 treatment. IFN-γ (A), TNF-α (B), simultaneous IFN-γ and TNF-α (C), and IL-2 (D) production in unstimulated (NS), Gag-peptide stimulated, and anti-CD3/CD28 stimulated CD8 + T cells is shown for each of three elite suppressors with the mean ± standard error. Significance was determined via a series of one-way repeated measures ANOVAs examining each of the twelve conditions separately, and level of significance indicated above a bar is in comparison to no treatment from the same stimulation. * p < 0.05. |
3.4. PKC agonist effects upon cell death and exhaustion in T cells
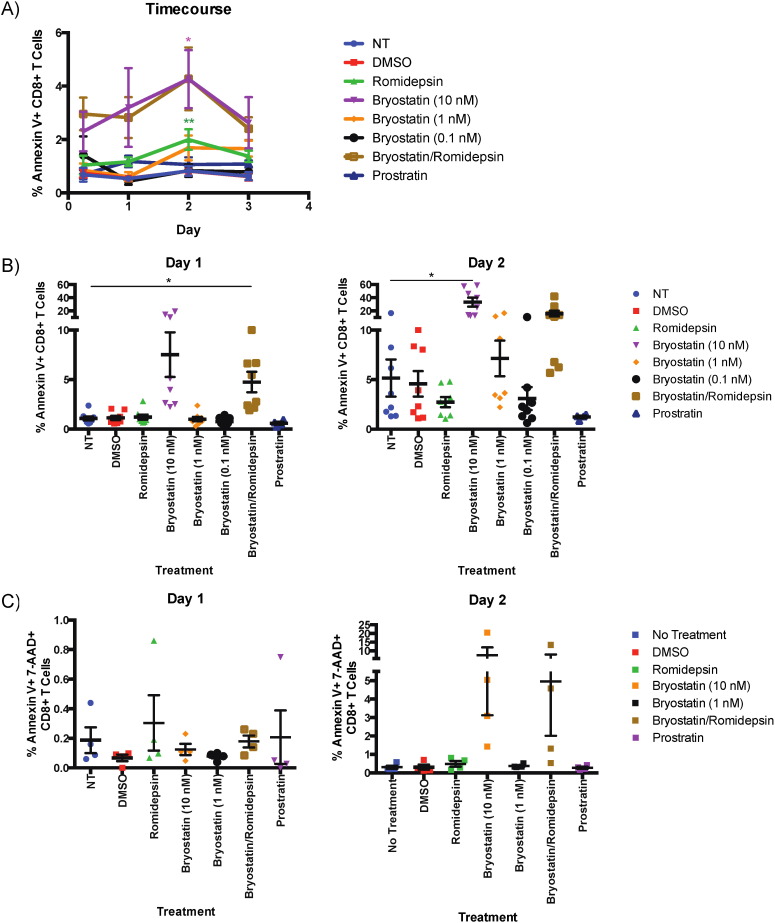
To further elucidate the mechanisms by which the LRAs affected CD8 + T cell function, CD8 + T cells from HIV-negative donors were interrogated for the amount of cell death induced by drug treatment as determined by Annexin V expression. For both 6-hour and 18-hour treatments with LRAs, the combination B/R treatment induced a trend towards a higher amount of cell death than cells treated with DMSO (not shown). To examine the effects of the LRAs on cell viability over time, cells were treated for six hours before being washed and cultured for an additional three days in the absence of stimulation. For this time course, 10 nM bryostatin-1-treated CD8 + T cells had a trend towards more cell death on day 1 and significantly more on day 2 (p < 0.05). A trend towards higher cell death was likewise observed in B/R -treated CD8 + T cells (Fig. 4 B). To model the effect of the LRAs on activated CD8 + T cells, CD8 + T cells treated with 10 nM bryostatin-1 and B/R for six hours were stimulated with anti-CD3/CD28 antibodies for an additional two days. Cells treated with anti-CD3/CD28 generally had increased cell death compared to CD8 + T cells that did not receive this treatment, but only bryostatin-1 and B/R treatment of anti-CD3 activated cells caused a significant increase in cell death compared to antibody treatment alone as determined by annexin V expression (Day 1, bryostatin-1: p < 0.05; Day 2, B/R: p < 0.05; Fig. 4 B) and supported by trends with annexin V and 7-AAD co-expression.
|
|
|
Fig. 4. Bryostatin-1 and bryostatin-1/romidepsin combination treatments cause an increase in CD8 + T cell death. Eight HIV negative donors' PBMCs were treated with LRAs and examined for annexin V expression on CD8 + T cells. A) Annexin V expression in CD8 + T cells treated with drug for 6 h and then cultured for three days post-treatment in non-stimulating media. B) Annexin V expression in CD8 + T cells treated with drug for 6 h and then stimulated with anti-CD3/CD28 for either one or two days afterward. C) Annexin V and 7-AAD expression in CD8 + T cells treated with drug for 6 h and then stimulated with anti-CD3/CD28 for either one or two days afterward. One-way repeated measures ANOVAs were used to calculate significance, and the level of significance indicated is in comparison to the no treatment condition. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. |
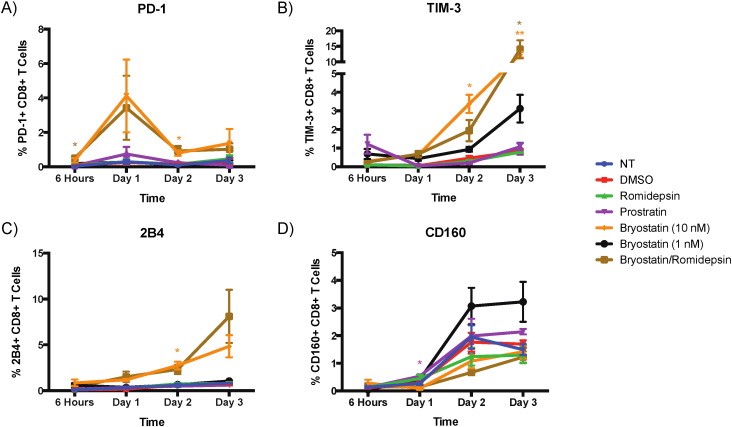
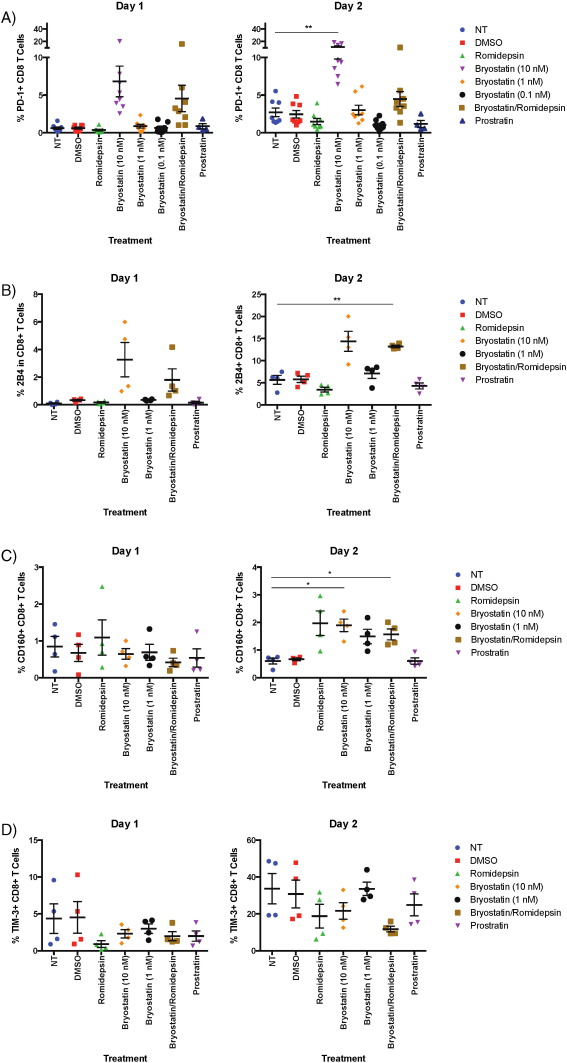
We also examined the expression of the exhaustion markers PD-1, Tim-3, 2B4, and CD160 following 6 h of treatment with the different drugs as a potential cause of the observed inhibition of CD8 + T cell responses. Bryostatin-1 at 10 nM and B/R induced modest but significant increases in PD-1, TIM-3 and 2B4 expression at different time points over a 3 day time period (example of PD-1 gating shown in Supplementary Fig. 1 A; Fig. 5 A–C). In order to determine the effects of the LRAs on activated cells, we looked at the expression of these exhaustion markers on cells that were exposed to drugs for 6 h and then stimulated with CD3 and CD28 specific antibodies. PD-1, CD160 and 2B4 expression levels were significantly increased in activated cells that were treated with bryostatin-1 or B/R on day 2 whereas romidepsin tended to increase CD160 expression on the activated cells at the same time point (Fig. 6 A–C). Furthermore, bryostatin-1 also significantly PD-1 expression in the stimulated cells on day 2 as measured by ratio of the mean fluorescence intensity of the treated cells to that of the untreated cells (p < 0.05; Supplementary Fig. 1 B).
|
|
|
Fig. 5. Bryostatin-1 treatment induces an increase in exhaustion marker expression in unstimulated CD8 + T cells. Eight HIV-negative donors' PBMCs were treated with LRAs and examined for PD-1 expression on CD8 + T cells, and four donors' PBMCs were examined for TIM-3, 2B4, and CD160 expression. A) PD-1 expression in CD8 + T cells treated with drug for 6 h and then incubated in non-stimulating media for an additional three days. B) TIM-3 expression. C) 2B4 expression. D) CD160 expression. The level of significance indicated is in comparison to no treatment. * p < 0.05, ** p < 0.01. |
|
|
|
Fig. 6. Bryostatin-1 treatment induces an increase in exhaustion marker expression in stimulated CD8 + T cells. Eight HIV-negative donors' PBMCs were treated with LRAs and examined for PD-1 expression on CD8 + T cells, and four donors' PBMCs were examined for TIM-3, 2B4, and CD160 expression. A) PD-1 expression in CD8 + T cells treated with drug for 6 h and then incubated with anti-CD3/CD28 antibodies for an additional two days. B) 2B4 expression. C) CD160 expression. D) TIM-3 expression. The level of significance indicated is in comparison to no treatment. * p < 0.05, ** p < 0.01. |
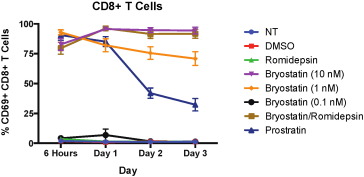
In order to determine whether the expression of the exhaustion markers was just a marker of T cell activation we looked at CD69 expression on CD8 + T cells treated with LRAs. Bryostatin-1 treatment has been shown to induce CD69 on resting CD4 + T cells (Laird et al., 2015 ), and we found very high levels of this early activation marker on CD8 + T cells that were treated with 10 nM and 1 nM bryostatin-1, B/R, and prostratin (Fig. 7 ). PD-1, 2B4 and TIM-3 expression was not upregulated on prostratin-treated cells, implying that the expression of these exhaustion markers was not just a reflection of partial activation although further experiments are needed to verify this.
|
|
|
Fig. 7. CD69 expression is upregulated due to treatment with PKC agonists. Eight HIV-negative donors' PBMCs were treated with LRAs for 6 h before being washed and then cultured in non-stimulating media for up to three days and examined for CD69 expression on CD8 + T cells. Mean expression ± standard error is indicated for each treatment. Bryostatin-1 treatment at 10 nM and 1 nM and bryostatin-1 (10 nM)/romidepsin treatment all significantly upregulate CD69 for all four timepoints (p < 0.0001), as did prostratin treatment (p < 0.001, p < 0.001, p < 0.01, and p < 0.05 at the respective timepoints) as calculated by multiple one-way repeated measures ANOVAs. |
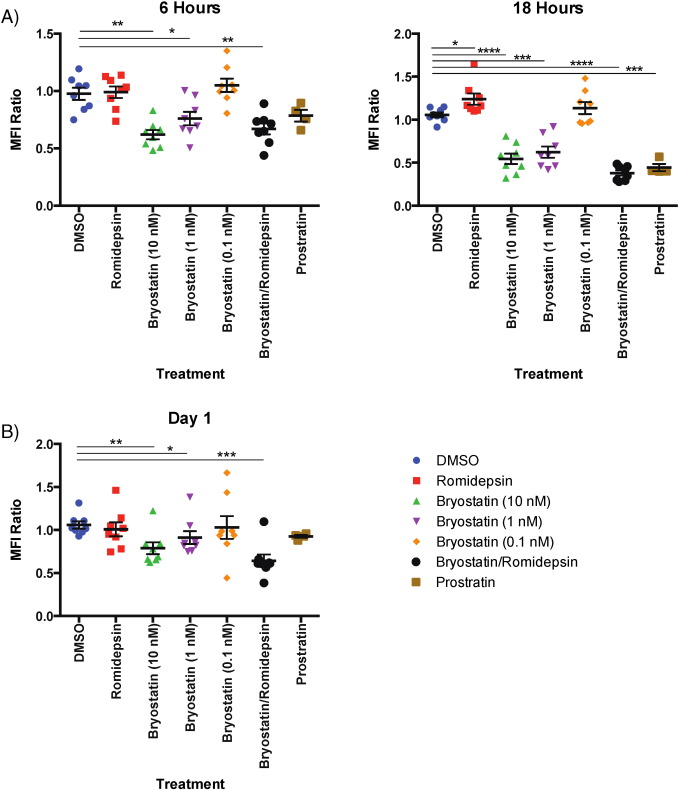
PKC agonists such as PMA have been known to downregulate CD3 (Salio et al., 1997 ), which has the potential to inhibit T cell responses. In order to determine whether LRA treatment had an effect on CD3 expression, we examined the change in the mean fluorescence intensity (MFI) of this marker. CD8 + T cells treated with bryostatin-1 at 10 nM and 1 nM and B/R had a significant decrease in CD3 expression after six hours of treatment (bryostatin-1: p < 0.01 at 10 nM, p < 0.05 at 1 nM; B/R: p < 0.01; Fig. 8 A). However, after 18 h of treatment, bryostatin-1 at 10 nM and 1 nM, the B/R combination, and prostratin alone cause significant decreases in CD3 expression compared to untreated cells (p < 0.0001 for 10 nM bryostatin-1 and B/R; p < 0.001 for bryostatin-1 at 1 nM and prostratin; Fig. 8 A). For CD8 + T cells treated for six hours and then cultured for a day in the absence of treatment, bryostatin-1 at 10 nM and 1 nM as well as B/R continued to cause a significant decrease in CD3 expression (bryostatin-1: p < 0.01, p < 0.05, respectively; B/R: p < 0.001; Fig. 8 B). Unlike bryostatin-1 treatment alone, however, the effect of B/R on CD3 expression maintained at day 1 (Fig. 8 A,B). Overall, while bryostatin-1 has an effect on CD3 expression alone, the combination of bryostatin-1 with romidepsin causes both a more severe and longer-lasting phenotype.
|
|
|
Fig. 8. CD3 expression is decreased due to treatment with bryostatin-1, prostratin, and the combination of bryostatin-1/romidepsin. Eight HIV-negative donors' PBMCs were treated with LRAs for at least 6 h and examined for CD3 expression on CD8 + T cells. A) Mean fluorescence intensity (MFI) of CD3 expression in CD8 + T cells treated with drug for 6 or 18 h normalized to no treatment. One-way repeated measures ANOVAs were used to calculate significance for 6-hour and 18-hour treatments separately. B) Mean fluorescence intensity (MFI) of CD3 expression in CD8 + T cells treated with drug for 6 h and then incubated in non-stimulating media for one day afterward normalized to no treatment. A one-way repeated measures ANOVA was used to calculate significance. The level of significance indicated is in comparison to vehicle (DMSO). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. |
4. Discussion
Current HIV-1 cure strategies seek to eliminate the latent reservoir by specifically activating HIV-1 so the immune system can clear the latently infected cells. The most promising latency reactivating agent (LRA) regimens in vitro thus far appear to be combinations of HDAC inhibitors (HDACi) and PKC agonists (Laird et al., 2015 ). However, some of these drugs have previously been suggested to have immunomodulatory effects (Jones et al., 2014 ; reviewed in Akimova et al., 2012 , and Remoli et al., 2012 ). In this study, we confirm that the combination of bryostatin-1 and romidepsin is effective in reversing latency, however we found that even following stimulation with high concentrations of IL-2 and Gag and Nef consensus peptides, CD8 + T cells from fully suppressed chronic progressors were unable to reduce the amount of HIV-1 mRNA associated with CD4 + T cells or prevent release of virions from these cells in the context of bryostatin-1 and romidepsin treatment. One limitation of our study is that we did not measure actual viral protein production or antigen presentation following latency reversal. It is possible that some of the mRNA we measured is defective and did not lead to the synthesis of functional proteins that could be recognized by CD8 + T cells. Another possibility is that the effects of the LRAs may be short lived in vivo since HIV-specific CD8 + T cells from patients treated with vorinostat (Sung et al., 2015 ) and romidepsin (Søgaard et al., 2015 ) appeared to be functional in ex vivo studies, but even a short term effect could be important for viral clearance. Another limitation is the relatively small number of patients studied in this manuscript.
In a prior study, autologous ex vivo expanded virus-specific cytototoxic T lymphocytes, but not unexpanded CD8 + T cells, from HIV infected patients were able to significantly reduce the number of latently infected cells following reversal with vorinostat (Sung et al., 2015 ). The discrepancy between that study and our findings could potentially be explained by the fact that vorinostat has much less of an effect of on CD8 + T cells than did romidepsin, bryostatin-1 and the combination of the 2 drugs. Chronic progressors' primary CD8 + T cells are generally not effective in controlling HIV-1 replication (reviewed in Migueles and Connors, 2015 ), which may also partially account for observed results. In order to determine other potential causes, we examined the effect of four separate LRAs alone and in combination on the HIV-specific CD8 + T cell response of elite suppressors.
Elite suppressors are HIV-positive individuals who have viral loads of < 50 copies of HIV-1 RNA per milliliter in the absence of antiretroviral therapy (O'Connell et al., 2009 ). These individuals are known to have qualitatively superior HIV-specific CD8 + T cell responses than chronic progressors (Betts et al., 2006 , Migueles et al., 2002 , Sáez-Cirión et al., 2007 , Migueles et al., 2008 and Hersperger et al., 2010 ). We performed suppression assays with cells from four elite suppressors and found that untreated CD8 + T cells were able to suppress infection effectively but that treatment with romidepsin (40 nM) or bryostatin-1 (10 nM) alone significantly inhibited this suppression by nearly 50%. The combination of the two LRAs at those concentrations fully ablated the suppression in two individuals and otherwise significantly reduced the average suppression by 90% suggesting that the two drugs may have an additive, negative effect (Fig. 2 A). Interestingly, prostratin, another PKC agonist did not have an inhibitory effect on CD8 + T cell mediated suppression, suggesting that the effect on CD8 + T cell function may not be a feature of the entire class of drugs. HDAC inhibitors such as romidepsin and panobinostat have been shown to selectively cause the death of activated cells (Jones et al., 2014 ), and PKC agonists such as bryostatin-1, prostratin, and PMA are known to cause partial activation in T cells (Hess et al., 1988 , Drexler et al., 1990 and Scheid et al., 1994 ), with bryostatin-1 specifically acting as a TLR-4 ligand (Ariza et al., 2011 ). We therefore combined romidepsin with prostratin, which also induced partial activation as determined by CD69 expression, to determine if the additive negative effect seen when bryostatin-1 and romidepsin were combined was a general effect of adding an HDAC inhibitor to a PKC agonist. Interestingly, the combination had an equal amount of suppression as romidepsin alone (Fig. 2 B). JQ1 (1 μM), a bromodomain-containing protein 4 inhibitor, also had minimal effect on CD8 + T cell mediated suppression and did not appear to have an additive inhibitory effect when it was combined with bryostatin-1. The combination of prostratin and JQ1 has also recently shown to be effective (Laird et al., 2015 ) and these drugs together did not have an adverse effect on CD8 + T cell function.
In order to determine the mechanism for the inhibition of suppression seen with romidepsin, bryostatin-1, and the combination of the two, we examined the LRA treatment-dependent toxicity and induction of exhaustion on CD8 + T cells. Bryostatin-1 treatment at 10 nM causes an increase in cell death and PD-1 expression, and combined bryostatin-1 and romidepsin treatment more closely mimics the effects of bryostatin-1 treatment as compared to romidepsin treatment, suggesting that the phenomena are bryostatin-1-mediated. The increased cell death and exhaustion shortly after treatment with 10 nM bryostatin-1 likely contributes to the CD8 + T cell dysfunction in the suppression assays, given that CD8 + T cell killing of HIV-1 infected CD4 + T cells can occur within an hour (Migueles et al., 2008 ).
PKC agonists are known to downregulate CD3 (Salio et al., 1997 ) as well as CD4 (Gulakowski et al., 1997 , Kulkosky et al., 2001 , Hezareh et al., 2004 and Warrilow et al., 2006 ). Downregulation of CD3-TCR complexes as well as the coreceptors CD4 and CD8 may inhibit the ability of effector T cells to respond to their cognate antigen, leading us to examine the expression of these markers in T cells. We found that bryostatin-1 treatment caused transient downregulation of CD3 in unstimulated CD8 + T cells, but treatment with bryostatin-1 and romidepsin prolonged this effect. Even a transient effect may be important because we have previously shown that virion release from latently infected CD4 + T cells may occur as early as 6 h after activation (Walker-Sperling et al., 2015 ), and therefore a quick immune response will be needed to eliminate reactivated CD4 + T cells.
In summary, we have shown that the HDACi/PKC agonist LRA combination of bryostatin-1 and romidepsin causes marked inhibition of the HIV-specific CD8 + T cell response, as do both drugs alone. The inhibition of the T cell response by bryostatin-1 may be due an increase in T cell death and exhaustion marker expression as well as a downregulation of CD3, resulting in a decreased ability of T cells to respond to stimuli. The combination of romidepsin with bryostatin-1 furthermore causes a more severe and possibly longer-lasting downregulation phenotype, potentially contributing to the more severe inhibition of the T cell response. Any HIV-1 cure strategy involving LRA-based reactivation will likely depend upon the immune response to eliminate any latently infected cells, but given the range of negative effects the LRAs have upon the immune response alone and in combination, each potential LRA therapy should be examined for its broad effects on adaptive immunity before use in the context of HIV-1 cure. Combinations of latency reversal agents such as prostratin and JQ1 that together do not have significant effects on HIV-specific immune responses may be the most effective candidates for the shock and kill approach to HIV-1 eradication.
The following is the supplementary data related to this article.
Supplementary Fig. 1.
Bulk CD8 + T cell expression of PD-1 is upregulated as measured by mean fluorescence intensity (MFI). Eight HIV-negative donor PBMCs were treated with LRAs for six hours prior to washing and further culture in the presence of anti-CD3/CD28 antibodies. A) Gating schematic for PD-1 as measured in Fig. 5 and Fig. 6 . B) Expression of PD-1 as calculated instead by the MFI of PD-1 in treated cells divided by the MFI of PD-1 in untreated cells. * p < 0.05.
Funding sources
Funded by the Johns Hopkins University Center for AIDS Research (P30AI094189 ) and 2R56AI080328-05A1 and 1R01AI120024-01 (JNB ). The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Conflict of interest
None of the authors have any conflicts of interest.
Author contributions
Victoria Walker-Sperling performed data collection, data analysis, data interpretation and wrote the paper.
Christopher Pohlmeyer performed data collection, data analysis and helped interpret the data.
Patrick Tarwater performed data analysis and helped interpret the data.
Joel Blankson designed the study, performed data analysis and interpretation and wrote the paper.
Acknowledgements
We thank Dr. Gregory Laird for advice and Yury Kuzmichev and Caroline Garliss for helpful discussions.
References
- Akimova et al., 2010 T. Akimova, G. Ge, T. Golovina, T. Mikheeva, L. Wang, J.L. Riley, W.W. Hancock; Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3 + Tregs; Clin. Immunol., 136 (3) (2010), pp. 348–363 http://dx.doi.org/10.1016/j.clim.2010.04.018
- Akimova et al., 2012 T. Akimova, U.H. Beier, Y. Liu, L. Wang, W.W. Hancock; Histone/protein deacetylases and T -cell immune responses ; Blood, 119 (11) (2012), pp. 2443–2451 http://dx.doi.org/10.1182/blood-2011-10-292003
- Archin and Margolis, 2014 N.M. Archin, D.M. Margolis; Emerging strategies to deplete the HIV reservoir; Curr. Opin. Infect. Dis., 27 (2014), pp. 29–35 http://dx.doi.org/10.1097/QCO.0000000000000026
- Archin et al., 2012 N.M. Archin, A.L. Liberty, A.D. Kashuba, S.K. Choudhary, J.D. Kuruc, A.M. Crooks, D.C. Parker, E.M. Anderson, M.F. Kearney, M.C. Strain, D.D. Richman, M.G. Hudgens, R.J. Bosch, J.M. Coffin, J.J. Eron, D.J. Hazuda, D.M. Margolis; Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy; Nature, 487 (7408) (2012), pp. 482–485
- Ariza et al., 2011 M.E. Ariza, R. Ramakrishnan, N.P. Singh, A. Chauhan, P.S. Nagarkatti, M. Nagarkatti; Bryostatin-1-1, a naturally occurring antineoplastic agent, acts as a toll-like receptor 4 (TLR-4) ligand and induces unique cytokines and chemokines in dendritic cells; J. Biol. Chem., 286 (1) (2011), pp. 24–34 http://dx.doi.org/10.1074/jbc.M110.135921
- Bauer et al., 2014 S. Bauer, R.A. Hilger, T. Mühlenberg, F. Grabellus, J. Nagarajah, M. Hoiczyk, A. Reichardt, M. Ahrens, P. Reichardt, S. Grunewald, M.E. Scheulen, A. Pustowka, E. Bock, M. Schuler, D. Pink; Phase I study of panobinostat and imatinib in patients with treatment-refractory metastatic gastrointestinal stromal tumors; Br. J. Cancer, 110 (5) (2014), pp. 1155–1162
- Betts et al., 2006 M.R. Betts, M.C. Nason, S.M. West, S.C. De Rosa, S.A. Migueles, J. Abraham, M.M. Lederman, J.M. Benito, P.A. Goepfert, M. Connors, M. Roederer, R.A. Koup; HIV nonprogressors preferentially maintain highly functional HIV-specific CD8 + T cells; Blood, 107 (2006), pp. 4781–4789
- Buckheit et al., 2012 R.W. Buckheit 3rd, M. Salgado, R.F. Silciano, J.N. Blankson; Inhibitory potential of subpopulations of CD8 + T cells in HIV-1-infected elite suppressors; J. Virol., 86 (24) (2012), pp. 13679–13688 http://dx.doi.org/10.1128/JVI.02439-12
- Bullen et al., 2014 C.K. Bullen, G.M. Laird, C.M. Durand, J.D. Siliciano, R.F. Siliciano; New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo; Nat. Med., 20 (4) (2014), pp. 425–429 http://dx.doi.org/10.1038/nm.3489
- Contreras et al., 2009 X. Contreras, M. Schweneker, C.S. Chen, J.M. McCune, S.G. Deeks, J. Martin, B.M. Peterlin; Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells; J. Biol. Chem., 284 (11) (2009), pp. 6782–6789 http://dx.doi.org/10.1074/jbc.M807898200
- DeChristopher et al., 2012 B.A. DeChristopher, B.A. Loy, M.D. Marsden, A.J. Schrier, J.A. Zack, P.A. Wender; Designed, synthetically accessible bryostatin-1 analogues potently induce activation of latent HIV reservoirs in vitro; Nat. Chem., 4 (9) (2012), pp. 705–710 http://dx.doi.org/10.1038/nchem.1395
- Drexler et al., 1990 H.G. Drexler, S.M. Gignac, G.R. Pettit, A.V. Hoffbrand; Synergistic action of calcium ionophore A23187 and protein kinase C activator bryostatin-1 on human B cell activation and proliferation; Eur. J. Immunol., 20 (1) (1990), pp. 119–127
- Darcis et al., 2015 G. Darcis, A. Kula, S. Bouchat, K. Fujinaga, F. Corazza, A. Ait-Ammar, N. Delacourt, A. Melard, K. Kabeya, C. Vanhulle, B. Van Driessche, Gatot JS, T. Cherrier, Pianowski LF, L. Gama, C. Schwartz, J. Vila, A. Burny, N. Clumeck, M. Moutschen, S. De Wit, Peterlin BM, C. Rouzioux, O. Rohr, C. Van Lint; An in-depth comparison of latency-reversing agent combinations in various in vitro and ex vivo HIV-1 latency models identified bryostatin-1-1 + JQ1 and ingenol-B + JQ1 to potently reactivate viral gene expression; PLoS Pathog., 11 (7) (2015), Article e1005063 http://dx.doi.org/10.1371/journal.ppat.1005063
- Gulakowski et al., 1997 R.J. Gulakowski, J.B. McMahon, R.W. Buckheit Jr., K.R. Gustafson, M.R. Boyd; Antireplicative and anticytopathic activities of prostratin, a non-tumor-promoting phorbol ester, against human immunodeficiency virus (HIV); Antivir. Res., 33 (2) (1997), pp. 87–97
- Hersperger et al., 2010 A.R. Hersperger, F. Pereyra, M. Nason, K. Demers, P. Sheth, L.Y. Shin, C.M. Kovacs, B. Rodriguez, S.F. Sieg, L. Teixeira-Johnson, D. Gudonis, P.A. Goepfert, M.M. Lederman, I. Frank, G. Makedonas, R. Kaul, B.D. Walker, M.R. Betts; Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control; PLoS Pathog., 6 (2010), Article e1000917 http://dx.doi.org/10.1371/journal.ppat.1000917
- Hess et al., 1988 A.D. Hess, M.K. Silanskis, A.H. Esa, G.R. Pettit, W.S. May; Activation of human T lymphocytes by bryostatin-1; J. Immunol., 141 (10) (1988), pp. 3263–3269
- Hezareh et al., 2004 M. Hezareh, M.A. Moukil, I. Szanto, M. Pondarzewski, S. Mouche, N. Cherix, S.J. Brown, J.L. Carpentier, M. Foti; Mechanisms of HIV receptor and co-receptor down-regulation by prostratin: role of conventional and novel PKC isoforms; Antivir. Chem. Chemother., 15 (4) (2004), pp. 207–222
- Jiang et al., 2015 G. Jiang, E.A. Mendes, P. Kaiser, D.P. Wong, Y. Tang, I. Cai, A. Fenton, G.P. Melcher, J.E. Hildreth, G.R. Thompson, J.K. Wong, S. Dandekar; Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p -TEFb activation ; PLoS Pathog., 11 (7) (2015), Article e1005066 http://dx.doi.org/10.1371/journal.ppat.1005066
- Jones et al., 2014 R.B. Jones, R. O'Connor, S. Mueller, M. Foley, G.L. Szeto, D. Karel, M. Lichterfeld, C. Kovacs, M.A. Ostrowski, A. Trocha, D.J. Irvine, B.D. Walker; Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes; PLoS Pathog., 10 (8) (2014), Article e1004287 http://dx.doi.org/10.1371/journal.ppat.1004287
- Kelly-Sell et al., 2012 M.J. Kelly-Sell, Y.H. Kim, S. Straus, B. Benoit, C. Harrison, K. Sutherland, R. Armstrong, W.K. Weng, L.C. Showe, M. Wysocka, A.H. Rook; The histone deacetylase inhibitor, romidepsin, suppresses cellular immune functions of cutaneous T-cell lymphoma patients; Am. J. Hematol., 87 (4) (2012), pp. 354–360 http://dx.doi.org/10.1002/ajh.23112
- Kulkosky et al., 2001 J. Kulkosky, D.M. Culnan, J. Roman, G. Dornadula, M. Schnell, M.R. Boyd, R.J. Pomerantz; Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART; Blood, 98 (10) (2001), pp. 3006–3015
- Laird et al., 2015 G.M. Laird, C.K. Bullen, D.I. Rosenbloom, A.R. Martin, A.L. Hill, C.M. Durand, J.D. Siliciano, R.F. Siliciano; Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations; J. Clin. Invest., 125 (5) (2015), pp. 1901–1912 http://dx.doi.org/10.1172/JCI80142
- Li et al., 2013 Z. Li, J. Guo, Y. Wu, Q. Zhou; The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of tat-transactivation; Nucleic Acids Res., 41 (1) (2013), pp. 277–287 http://dx.doi.org/10.1093/nar/gks976
- Migueles and Connors, 2015 S.A. Migueles, M. Connors; Success and failure of the cellular immune response against HIV-1; Nat. Immunol., 16 (6) (2015), pp. 563–570 http://dx.doi.org/10.1038/ni.3161
- Migueles et al., 2002 S.A. Migueles, A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, M. Connors; HIV-specific CD8 + T cell proliferation is coupled to perforin expression and is maintained in nonprogressors; Nat. Immunol., 3 (2002), pp. 1061–1068
- Migueles et al., 2008 S.A. Migueles, C.M. Osborne, C. Royce, A.A. Compton, R.P. Joshi, K.A. Weeks, J.E. Rood, A.M. Berkley, J.B. Sacha, N.A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M.A. O'Shea, J. Mican, B.Z. Packard, A. Komoriya, S. Palmer, A.P. Wiegand, F. Maldarelli, J.M. Coffin, J.W. Mellors, C.W. Hallahan, D.A. Follman, M. Connors; Lytic granule loading of CD8 + T cells is required for HIV-infected cell elimination associated with immune control; Immunity, 29 (2008), pp. 1009–1021 http://dx.doi.org/10.1016/j.immuni.2008.10.010
- O'Connell et al., 2009 K.A. O'Connell, J.R. Bailey, J.N. Blankson; Elucidating the elite: mechanisms of control in HIV-1 infection; Trends Pharmacol. Sci., 30 (12) (2009), pp. 631–637 http://dx.doi.org/10.1016/j.tips.2009.09.005
- Pohlmeyer et al., 2013 C.W. Pohlmeyer, R.W. Buckheit 3rd, R.F. Siliciano, J.N. Blankson; CD8 + T cells from HLA-B*57 elite suppressors effectively suppress replication of HIV-1 escape mutants; Retrovirology, 10 (2013), p. 152 http://dx.doi.org/10.1186/1742-4690-10-152
- Remoli et al., 2012 A.L. Remoli, G. Marsili, A. Battistini, M. Sgarbanti; The development of immune-modulating compounds to disrupt HIV latency; Cytokine Growth Factor Rev., 23 (4–5) (2012), pp. 159–172 http://dx.doi.org/10.1016/j.cytogfr.2012.05.003
- Rubin et al., 2006 E.H. Rubin, N.G. Agrawal, E.J. Friedman, P. Scott, K.E. Mazina, L. Sun, L. Du, J.L. Ricker, S.R. Frankel, K.M. Gottesdiener, J.A. Wagner, M. Iwamoto; A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer; Clin. Cancer Res., 12 (23) (2006), pp. 7039–7045
- Sáez-Cirión et al., 2007 A. Sáez-Cirión, C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barré-Sinoussi, J.F. Delfraissy, M. Sinet, G. Pancino, A. Venet, Agence Nationale de Recherches sur le Sida EP36 HIV Controllers Study Group; HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype; Proc. Natl. Acad. Sci. U. S. A., 104 (2007), pp. 6776–6781
- Salio et al., 1997 M. Salio, S. Valitutti, A. Lanzavecchia; Agonist-induced T cell receptor down-regulation: molecular requirements and dissociation from T cell activation; Eur. J. Immunol., 27 (7) (1997), pp. 1769–1773
- Scheid et al., 1994 C. Scheid, R. Young, R. McDermott, L. Fitzsimmons, J.H. Scarffe, P.L. Stern; Immune function of patients receiving recombinant human interleukin-6 (IL-6) in a phase I clinical study: induction of C-reactive protein and IgE and inhibition of natural killer and lymphokine-activated killer cell activity; Cancer Immunol. Immunother., 38 (2) (1994), pp. 119–126
- Shan et al., 2012 L. Shan, K. Deng, N.S. Shroff, C.M. Durand, S.A. Rabi, H.C. Yang, H. Zhang, J.B. Margolick, J.N. Blankson, R.F. Siliciano; Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation; Immunity, 36 (3) (2012), pp. 491–501 http://dx.doi.org/10.1016/j.immuni.2012.01.014
- Siliciano and Siliciano, 2013 J.D. Siliciano, R.F. Siliciano; HIV-1 eradication strategies: design and assessment; Curr. Opin. HIV AIDS, 8 (2013), pp. 318–325 http://dx.doi.org/10.1097/COH.0b013e328361eaca
- Smith et al., 2011 B.D. Smith, R.J. Jones, E. Cho, J. Kowalski, J.E. Karp, S.D. Gore, M. Vala, B. Meade, S.D. Baker, M. Zhao, S. Piantadosi, Z. Zhang, G. Blumenthal, E.D. Warlick, R.A. Brodsky, A. Murgo, M.A. Rudek, W.H. Matsui; Differentiation therapy in poor risk myeloid malignancies: results of a dose finding study of the combination bryostatin-1 and GM-CSF; Leuk. Res., 35 (1) (2011), pp. 87–94 http://dx.doi.org/10.1016/j.leukres.2010.06.001
- Søgaard et al., 2015 O.S. Søgaard, M.E. Graversen, S. Leth, R. Olesen, C.R. Brinkmann, S.K. Nissen, A.S. Kjaer, M.H. Schleimann, P.W. Denton, W.J. Hey-Cunningham, K.K. Koelsch, G. Pantaleo, K. Krogsgaard, M. Sommerfelt, R. Fromentin, N. Chomont, T.A. Rasmussen, L. Østergaard, M. Tolstrup; The depsipeptide romidepsin reverses HIV-1 latency in vivo; PLoS Pathog., 11 (9) (2015), Article e1005142
- Spivak et al., 2015 A.M. Spivak, A. Bosque, A.H. Balch, D. Smyth, L. Martins, V. Planelles; Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4(+) T cells from aviremic patients; Antimicrob. Agents Chemother., 59 (10) (2015), pp. 5984–5991 http://dx.doi.org/10.1128/AAC.01077-15
- Sung et al., 2015 J.A. Sung, S. Lam, C. Garrido, N. Archin, C.M. Rooney, C.M. Bollard, D.M. Margolis; Expanded cytotoxic T-cell lymphocytes target the latent HIV reservoir; J. Infect. Dis., 212 (2) (2015), pp. 258–263 http://dx.doi.org/10.1093/infdis/jiv022
- Tao et al., 2007 R. Tao, E.F. de Zoeten, E. Ozkaynak, C. Chen, L. Wang, P.M. Porrett, B. Li, L.A. Turka, E.N. Olson, M.I. Greene, A.D. Wells, W.W. Hancock; Deacetylase inhibition promotes the generation and function of regulatory T cells; Nat. Med., 13 (11) (2007), pp. 1299–1307
- Walker-Sperling et al., 2015 V.E. Walker-Sperling, V.J. Cohen, P.M. Tarwater, J.N. Blankson; Reactivation kinetics of HIV-1 and susceptibility of reactivated latently infected CD4 + T cells to HIV-1-specific CD8 + T cells; J. Virol., 89 (18) (2015), pp. 9631–9638 http://dx.doi.org/10.1128/JVI.01454-15
- Warrilow et al., 2006 D. Warrilow, J. Gardner, G.A. Darnell, A. Suhrbier, D. Harrich; HIV type 1 inhibition by protein kinase C modulatory compounds; AIDS Res. Hum. Retrovir., 22 (9) (2006), pp. 854–864
- Wei et al., 2014 D.G. Wei, V. Chiang, E. Fyne, M. Balakrishnan, T. Barnes, M. Graupe, J. Hesselgesser, A. Irrinki, J.P. Murry, G. Stepan, K.M. Stray, A. Tsai, H. Yu, J. Spindler, M. Kearney, C.A. Spina, D. McMahon, J. Lalezari, D. Sloan, J. Mellors, R. Geleziunas, T. Cihlar; Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing; PLoS Pathog., 10 (4) (2014), Article e1004071 http://dx.doi.org/10.1371/journal.ppat.1004071
- Williams et al., 2004 S.A. Williams, L.F. Chen, H. Kwon, D. Fenard, D. Bisgrove, E. Verdin, W.C. Greene; Prostratin antagonizes HIV latency by activating NF-kappaB; J. Biol. Chem., 279 (40) (2004), pp. 42008–42017
- Xing et al., 2011 S. Xing, C.K. Bullen, N.S. Shroff, L. Shan, H.C. Yang, J.L. Manucci, S. Bhat, H. Zhang, J.B. Margolick, T.C. Quinn, D.M. Margolis, J.D. Siliciano, R.F. Siliciano; Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4 + T cell model without inducing global T cell activation; J. Virol., 85 (12) (2011), pp. 6060–6064 http://dx.doi.org/10.1128/JVI.02033-10
Document information
Published on 06/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?