(Created page with " ==Abstract== Subcutaneous fluid administration is a commonly used therapy in veterinary practice. Its safety and efficacy have been demonstrated in human clinical studies,...") |
m (Scipediacontent moved page Draft Content 318412825 to Smith Greer 2016a) |
(No difference)
| |
Latest revision as of 10:27, 9 June 2017
Abstract
Subcutaneous fluid administration is a commonly used therapy in veterinary practice. Its safety and efficacy have been demonstrated in human clinical studies, but have only rarely been discussed in the veterinary literature. This prospective observational study was performed to evaluate changes during a 24 h period in serum biochemistries associated with administration of lactated Ringers solution subcutaneously to healthy cats. Lactated Ringers solution was administered subcutaneously once to ten healthy, euvolemic cats at a dose of 22 mL kg−1. Blood biochemistry analytes were sampled at baseline and at serial time points for a total of 24 h. Changes in biochemical analytes at each time point were compared to baseline and evaluated for statistical significance. Serum blood urea nitrogen (BUN) was significantly less than baseline at 4, 6, 12, 18, and 24 h post-infusion. Serum creatinine was significantly less than baseline at 2, 4 and 6 h. Packed cell volume (PCV) was significantly less than baseline at 6, 12, 18, and 24 h. Total plasma proteins were significantly less than baseline at all time points. Serum electrolytes did not change from baseline at any time point. Urine specific gravity was significantly increased from baseline only at 6 h post-Lactated Ringers solution (LRS) administration. Subcutaneous administration of lactated Ringers solution appears to result in haemodilution with minimal change to serum electrolyte concentrations in clinically normal, euvolemic cats.
Introduction
Administration of fluids subcutaneously (SCF), known also as hypodermoclysis, is a frequently used therapeutic option in mild to moderately dehydrated patients. Interest in hypodermoclysis has only recently been renewed in pediatric and geriatric human patients and in patients where venous access is difficult. In veterinary medicine, it is commonly used as an outpatient treatment, particularly in the emergency setting and in the chronic management of kidney disease.
When given intravenously, isotonic crystalloid administration has predominantly demonstrated effective volume expansive effects in veterinary studies. The described biochemical changes include reductions in packed cell volume (PCV), total plasma proteins (TP), and haemoglobin content which have been presumed as subsequent to haemodilution (Tollofsrud et al. 2001; Fielding et al. 2012; Valverde et al. 2008; Muir et al. 2011; Rose et al. 1980). Results of multiple studies involving modest to large infusion volumes have consistently found no changes in serum sodium or calcium. (Tollofsrud et al. 2001; Fielding et al. 2012; Valverde et al. 2008; Rose et al. 1980; Rohrig 2012; Rohrig et al. 2014). Changes in potassium have varied between null (Tollofsrud et al. 2001; Fielding et al. 2012; Rose et al. 1980; Rohrig et al. 2014) to increased (Valverde et al. 2008; Rohrig 2012) with intravenous infusion.
The efficacy and safety of SCF administration has been demonstrated in human medicine in a variety of clinical circumstances (Slesak et al. 2003; Lipschitz et al. 1991; O'Keeffe & Lavan 1996; Noriega & Blasco 2014). Biochemical changes associated with SCF administration in humans have been described, including haemodilution and reductions in urea nitrogen, creatinine, and osmolarity (Slesak et al. 2003; O'Keeffe & Lavan 1996; Noriega & Blasco 2014). Hyponatremia, usually mild, has also been described infrequently as an adverse effect of SCF administration (Slesak et al. 2003; Challiner et al. 1994). These findings parallel the changes described as a result of intravenous infusions of isotonic crystalloids in humans (Williams et al. 1999; Paydar et al. 2014; Lahsaee et al. 2013).
In contrast to human medicine, there is little published data on the pharmacokinetics and effects of subcutaneous fluid administration in veterinary patients. An earlier study of the effects of SCF in veterinary patients (Zontine & Donovan 1969) described a transient increase in PCV, TP, and chloride with the use of 5% dextrose in water (D5W). More recently, severe hyponatremia as a complication of SCF infusion has been described in two case reports (Lee et al. 2013; Evenchen & Aroch 2010) in cats. Otherwise, specific effects with respect to biochemical changes after SCF infusion have not been well described in veterinary patients. Thus, practices described in the current literature are largely a product of experience, anecdotal evidence, and extrapolation from human medicine.
The purpose of this study was to prospectively characterize the serum biochemical changes over a 24 h period associated with subcutaneously administered lactated Ringers solution (LRS) in euvolemic cats.
Materials and Methods
Ten clinically normal domestic shorthair cats with no history of recent or chronic illness were enrolled in the study. All cats were owned by employees of a private veterinary specialty hospital and were enrolled voluntarily with informed consent, as per institutional guidelines. All cats were housed individually at the veterinary hospital and provided with measured quantities of water and commercially produced dry maintenance food. Non-absorbent litter was provided at all times and was changed as needed after voids.
Experimental design
All cats were evaluated via physical examination, blood chemistry, complete blood count and urinalysis prior to initiation of the study. After initial evaluation, LRS (Lactated Ringers solution, Abbott Laboratories Inc., Chicago, IL). was administered to all patients over a 10-min period in accordance with previously described methods (Schaer 1989), using an 18 gauge needle inserted into the intrascapular subcutaneous tissues. The SCF dose administered was determined based on a typical feline body weight between 4.5 to 6.8 kg (10–15 pounds respectively) and was extrapolated from the most commonly cited dose in the current veterinary literature of 100–150 mL per cat (DiBartola & Bateman 2012; Polzin et al. 2005; Langston 2009). The dose is roughly equivalent to 22 mL kg−1 of measured body weight (10 mL lb−1), which is commonly utilized in clinical practice. The cats were briefly physically restrained for sampling purposes. Body weight was serially recorded, and packed cell volume (PCV), total plasma proteins (TP), blood urea nitrogen (BUN), creatinine, sodium, potassium, phosphorous, and calcium were sampled via peripheral venipuncture at baseline and at 2, 4, 6, 12, 18 and 24 h after fluid administration. All blood analyses were performed using centrifuged serum from tubes with no additives. Samples for urine specific gravity (USG) were collected via either cystocentesis or voided sample, at baseline and at 6, 12, 18 and 24 h after fluid administration. All samples were submitted to a regional reference laboratory (IDEXX Laboratories, Saint Louis, MO) for processing within 24 h of collection.
Statistical analysis
Serial measurements of individual patients were expected to be correlated. The correlation structure used for analysis of the data was a generalization of the autoregressive model, specifically the Mixed procedure. For each analyte, the residuals from the model were examined and were checked for normality using the Shapiro–Wilk test. Comparisons of the mean response at each time point to that of baseline for each measured analyte were done using Least Squares Means. Dunnetts method was used to adjust for multiple comparisons. Adjusted P-values of <0.05 were considered significant. Summary information is reported as mean ± standard deviation unless the values were skewed, in which case they are reported as median (range). The data are reported with a 95% confidence interval. All data were analysed using commercially available software (SAS, version 9.0, SAS Institute Inc., Cary, NC).
Results
A total of 10 cats were evaluated for the study, including 5 males and 5 females. All cats were neutered with a median age of 3 years (range 1–10 years). The median body weight was 5.0 kg (range 4.2–8.0 kg). One female cat was excluded from analysis due to renal insufficiency on baseline biochemistry as evidenced by concurrent azotemia and low urine specific gravity. There were no significant differences between patient values at baseline. Body weight was significantly increased from baseline at 2 and 4 h post–infusion, and then returned to baseline.
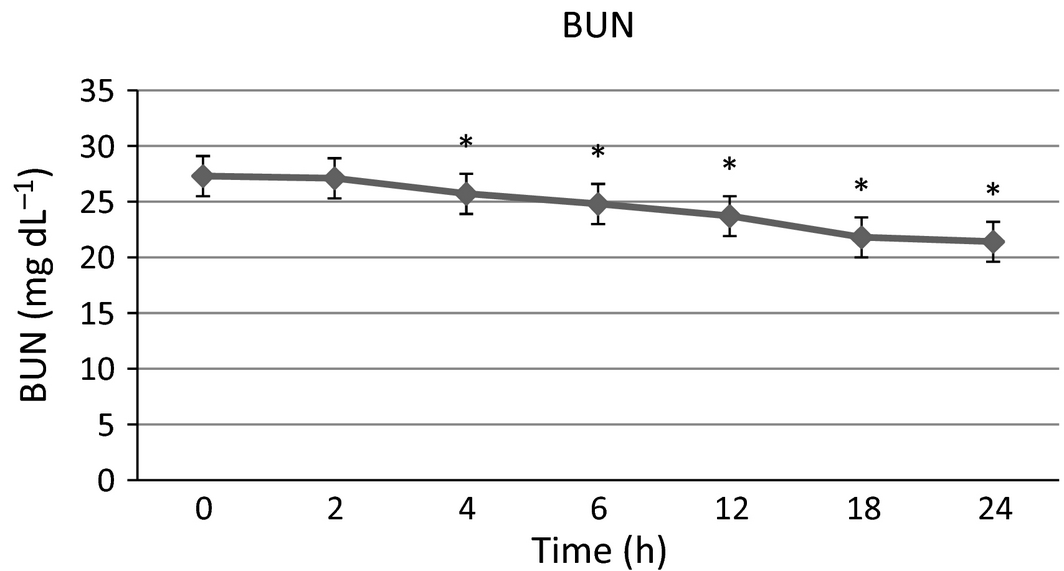
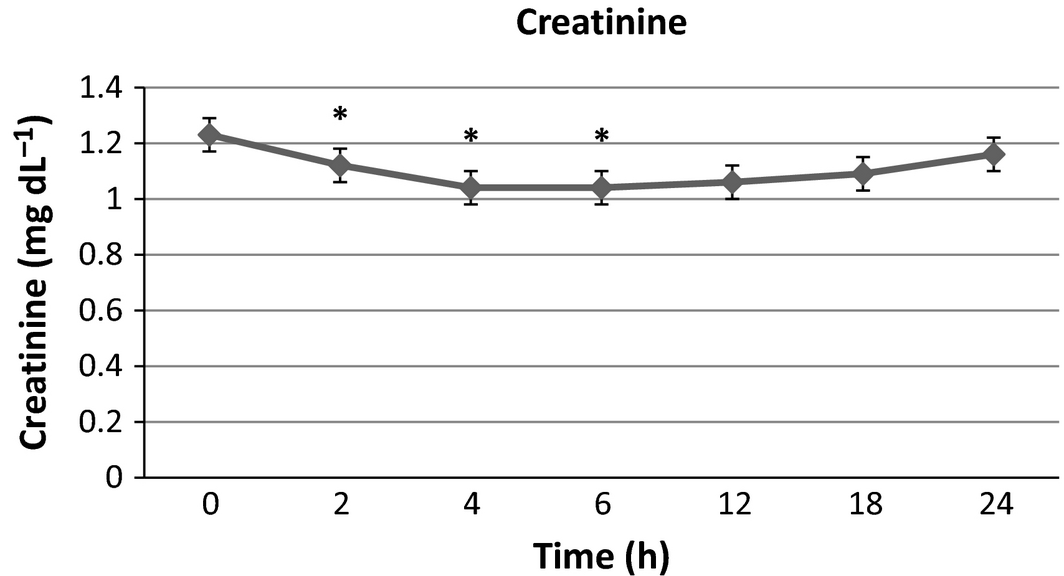
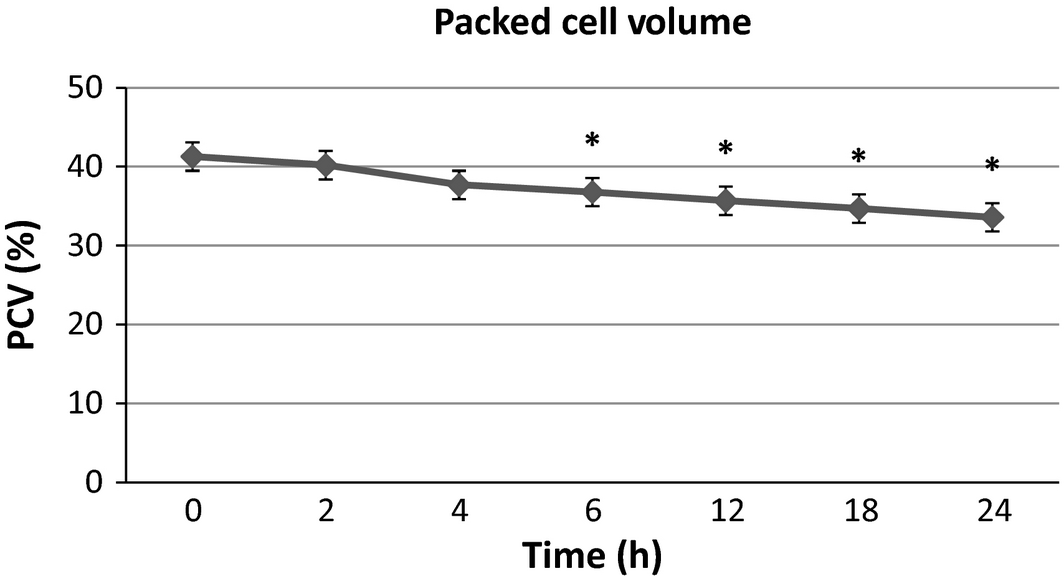
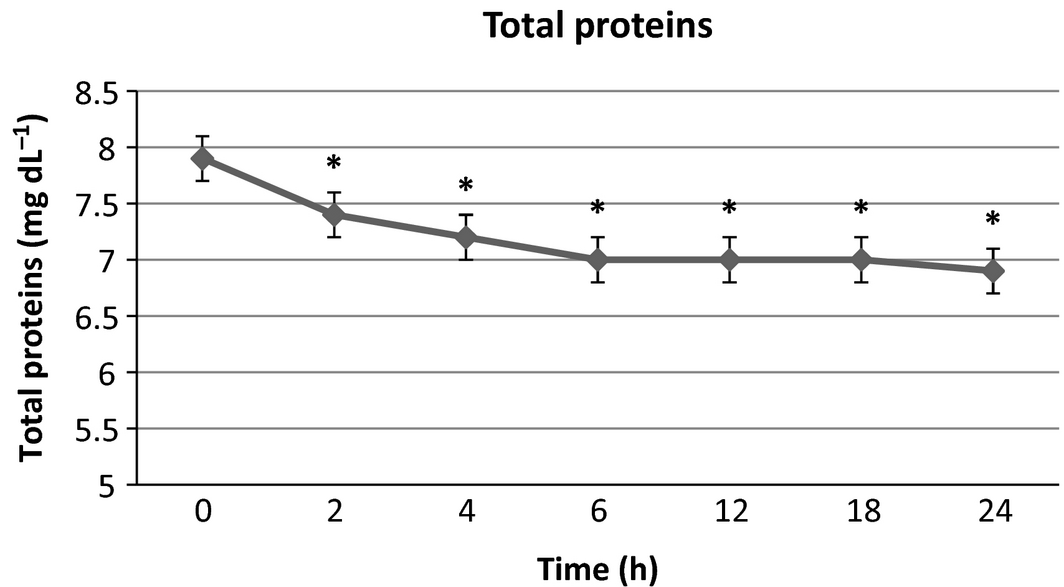
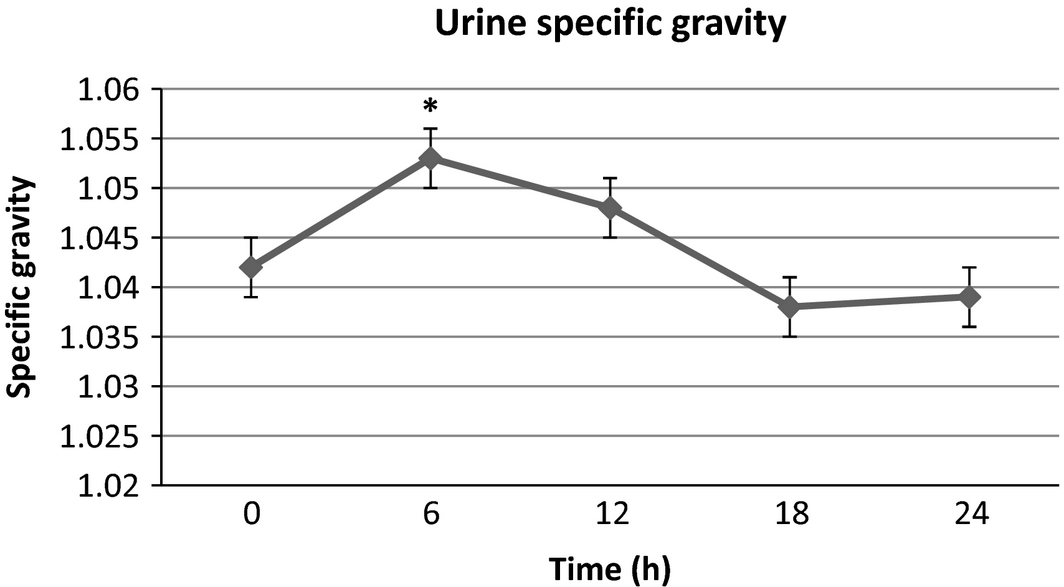
There were no significant differences in serum sodium, potassium and phosphorus from baseline at any time point. Blood urea nitrogen (Fig. 1) was significantly lower than baseline at 4, 6, 12, 18 and 24 h post-infusion with a maximal 21.6% decrease from baseline at the final time point. Creatinine (Fig. 2) was significantly lower than baseline at 2, 4 and 6 h with a maximal 15.4% decrease at 4–6 h after infusion, then trending towards baseline by the end of the study time period. Packed cell volume (Fig. 3) was significantly lower than baseline at 6, 12, 18 and 24 h with a maximal 18.6% decrease from the baseline at the final time point. Total proteins (TB) (Fig. 4) were significantly lower than baseline at all time points with a maximal 12.7% decrease from baseline at the final time point. Urine-specific gravity (Fig. 5) was significantly different from baseline only at 6 h post-infusion.
|
|
|
Figure 1. Blood urea nitrogen mean values over time +/− SD. * indicates significantly different from baseline (P < 0.05). |
|
|
|
Figure 2. Serum creatinine mean values over time +/− SD. * indicates significantly different from baseline (P < 0.05). |
|
|
|
Figure 3. Packed cell volume mean values over time +/− SD. * indicates significantly different from baseline (P < 0.05). |
|
|
|
Figure 4. Total protein mean values over time +/− SD. * indicates significantly different from baseline (P < 0.05). |
|
|
|
Figure 5. Urine specific gravity mean values over time +/− SD. * indicates significantly different from baseline (P < 0.05). |
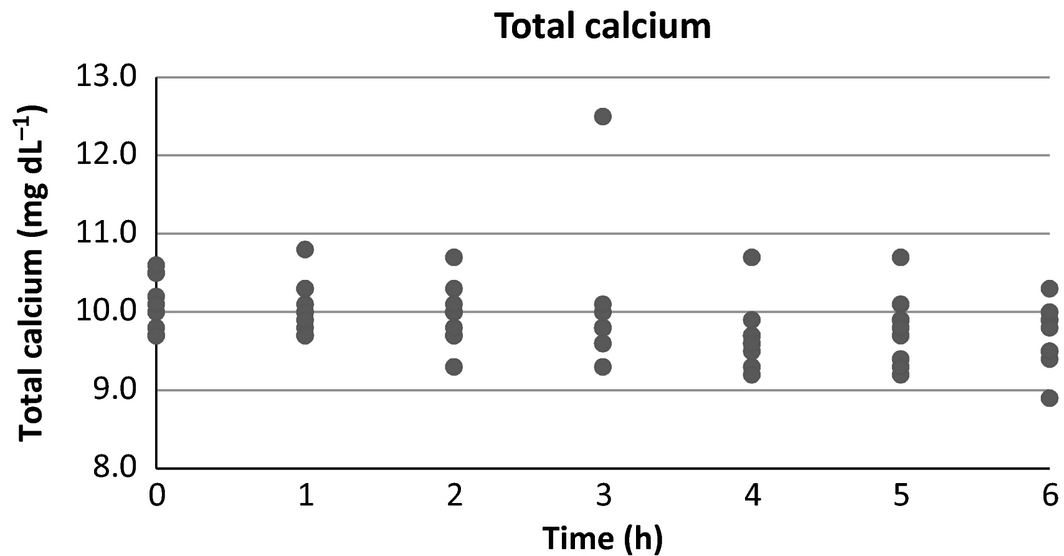
Serum calcium (Fig. 6) displayed an overall downward trend that did not reach statistical significance from baseline (P = 0.193) at any time point in this study. A single outlier was present at the 6-h time point which, if removed, would result in significance (P = 0.019) for that time point.
|
|
|
Figure 6. Serum calcium scatter plot over time. |
Discussion
This study was designed to determine biochemical changes in serum associated with subcutaneous administration of LRS in a healthy feline population. LRS was selected due to its isotonic nature, wide availability, electrolytic similarity to normal serum, and its frequent use at this institution. Additionally, a recent study in humans (Dychter et al. 2009) showed less discomfort and fewer adverse events associated with LRS compared with normal saline administered subcutaneously in humans. These findings were suspected to be due to pH and buffer differences between formulations.
In this study, both the PCV and TP decreased from baseline by 18.6% and 12.7% respectively. Both showed a progressive downward trend to the last time point in the study. These findings are in contrast to an earlier study (Zontine & Donovan 1969) in which D5W was administered subcutaneously and yielded an acute increase in these values, which was suspected to be secondary to transient plasma volume depletion due to fluid and electrolyte shifts from the vascular space to the interstitium as necessary to obtain ionic equilibrium with the electrolyte-devoid D5W. The decreases are in agreement with what has been seen in some clinical studies (Valverde et al. 2008; Muir et al. 2011; Wright & Hopkins 2008) of dogs receiving LRS boluses intravenously, which have been attributed to haemodilution from direct plasma volume expansion. Other similar studies (Gaynor et al. 1996) reported no change in PCV or TP attributed to fluid infusions. In each of the intravenous fluid studies, it should be noted that patients were under anaesthesia, which may itself cause transient decreases in PCV and TP (Wright & Hopkins 2008). The persistence of the PCV and TP decline up to the end of the sampling time of our study could have been caused by delayed or prolonged absorption of the infusate due to euvolemia. Further studies would be needed to determine if that trend would have continued beyond the current study parameters and to what extent.
Creatinine and BUN decreased from baseline by a maximum of 15.4% and 21.6% respectively in this study. The general decreases in BUN and creatinine over time are in accordance with the findings seen in human studies (Slesak et al. 2003; O'Keeffe & Lavan 1996; Noriega & Blasco 2014) evaluating biochemical changes and efficacy of SCF administration. To the authors' knowledge, this is the first study to directly report on effects of SCF administration on renal parameters over time in veterinary patients. The BUN followed a similar progressive trend to the PCV and TP in our study, thus these changes are suspected to also be due to plasma volume expansion and haemodilution. In this study, the serum creatinine did not fit this pattern and trended towards baseline after a trough at 4–6 h post-infusion. If consideration is given to an initial plasma volume expansion and subsequent recession as the volume is gradually redistributed, one would expect a similar trend towards baseline with all of the previously mentioned analytes. The exact mechanism of the findings with creatinine compared to the BUN, PCV and TP is unclear and would require further studies. The USG in this study was higher than baseline at 6 h then returned to baseline which does not support this theory, however, since sampling did not result in complete urine voids, the USG values beyond baseline may not have represented the true state of urine concentration at each time point. Additional studies would be necessary to definitively elucidate the mechanisms responsible for these findings. The possibility of contributions by increased creatinine production from a cause not identified during this study also cannot be ruled out.
There were no significant differences in serum sodium, potassium, or phosphorous in the present study. To the authors' knowledge, this is the first study to prospectively report electrolyte changes after SCF administration. Two recent case reports (Lee et al. 2013; Evenchen & Aroch 2010) described acute severe hyponatremia associated with subcutaneous infusions in a total of three cats. One cat (Lee et al. 2013) was administered a combination of 42.6 mL/kg LRS subcutaneously and 20 mL h−1 of water via an oesophagostomy tube prior to development of signs. The remaining two cats (Lee et al. 2013; Evenchen & Aroch 2010) received D5W subcutaneously at 40 mL kg−1 and 100 mL kg−1. In each of these reports, it was postulated that overzealous administration with electrolyte-free solutions was responsible for the hyponatremia. Our findings are consistent with studies in anaesthetized dogs and sheep receiving LRS boluses intravenously (Tollofsrud et al. 2001; Valverde et al. 2008; Muir et al. 2011; Rose et al. 1980) in which no alterations in serum electrolytes were seen. The lack of change in electrolytes is likely due to the similar electrolyte composition of LRS compared to serum.
Serum calcium was not found to be significantly different from baseline in this study. No prior studies of the effects of SCF administration on serum calcium concentration have been reported. Volume expansion and subsequent increase in glomerular filtration rate would be expected to induce some degree of calciuresis (Schenck et al. 2012). When added to the effect of haemodilution, a resultant difference in serum calcium may be expected, however, this has been an inconsistent finding in a previous study of hypercalcemic dogs (Rosol et al. 1994). The degree of change noted in these studies, however, may not be comparable to effects seen in healthy veterinary patients. Also, the presence of calcium in LRS (3 mEq L−1) may have precluded significant changes in total serum calcium in the present study. Further evaluation is necessary to more conclusively determine the expected changes in serum calcium with subcutaneous fluid therapy.
The present study has multiple important limitations to be considered. First, the limited sample size may have been too small to detect significant differences in some analytes. No control group was included in this study, thus normal variations in the measured parameters over time could not be used for comparison. Also, per os offering of food and water through the course of the study could have skewed the measurements, though it is considered marginal because no cats were observed eating or drinking for the duration of the study. Lastly, it is important to consider that the findings presented in this study are in healthy euvolemic patients. The changes seen here are not significant enough to be clinically relevant, but could be indicative of a generalized trend that may be of relevance to ill patients, particularly those with renal disease. Further studies are needed in such a population to definitively determine this effect.
Conclusions
Administration of LRS subcutaneously results in decreased PCV, TP, and BUN over a period lasting at least 24 h in healthy, euvolemic cats. A decrease in creatinine is also seen peaking at 4–6 h post-infusion. Subcutaneous infusion of LRS does not result in a difference in serum electrolyte concentrations in this patient population. Further studies on a larger scale will be required to confirm these findings and to establish a definitive length of time for the effects observed. Additional evaluation will also be necessary to illustrate differences, if any, in an ill patient population.
Acknowledgement
The authors thank Richard Madsen, PhD for aid in statistical analysis and IDEXX Laboratories for providing assistance and support for appropriate lab analysis.
Source of funding
This was supported by IDEXX Laboratories for providing assistance and support for appropriate lab analysis.
Conflicts of Interest
The authors declare that they have no conflicts of interest
Contributions
No additional contributions were made to this study.
References
- Challiner Y.C., Jarrett D., Hayward M.J., Al-Jubouri M.A. & Julious S.A. (1994) A comparison of intravenous and subcutaneous hydration in elderly acute stroke patients. Postgraduate Medical Journal70, 195–197.
- DiBartola S.P. & Bateman S. (2012) Introduction to fluid therapy. In: Fluid, electrolyte and acid-base disorders in small animal practice. 4th edn, 331–350. (ed S.P. DiBartola), Saunders: Philadelphia.
- Dychter S.S., Ebel B.S., Mead T.R. & Yocum R.C. (2009) Comparison of the tolerability of recombinant human hyaluronidase + normal saline and recombinant human hyaluronidase + lactated Ringers solution administered subcutaneously: A phase IV, double-blind, randomized pilot study in healthy volunteers. Current therapeutic research, clinical and experimental70, 421–438.
- Evenchen E. & Aroch I. (2010) Neurological abnormalities due to acute, severe hyponatremia in a kitten associated with subcutaneous infusion of 5% dextrose in water. Israel Journal of Veterinary Medicine65, 161–166.
- Fielding C.L., Magdesian K.G., Meier C.A. & Rhodes D.M. (2012) Clinical, hematological, and electrolyte changes with 0.9% sodium chloride or acetated fluids in endurance horses. Journal of Veterinary Emergency & Critical Care22, 327–331.
- Gaynor J.S., Wertz E.M., Kesel L.M., Baker G.E., Cecchini C., Rice K. & Mallinckrodt C.M. (1996) Effect of intravenous administration of fluids on packed cell volume, blood pressure, and total protein and blood glucose concentrations in healthy halothane-anesthetized dogs. Journal of the American Veterinary Medical Association208, 2013–2015.
- Lahsaee S.M., Ghaffaripour S. & Hejr H. (2013) The effect of routine maintenance intravenous therapy on hemoglobin concentration and hematocrit during anesthesia in adults. Bulletin of Emergency and Trauma1, 102–107.
- Langston C.E. (2009) Chronic renal failure. In: Small Animal Critical Care Medicine. 1st edn, 594–598. (eds D.C. Silversteine & K. Hopper), Saunders: Philadelphia.
- Lee J.Y., Rozanski E., Anastasio M., et al. (2013) Iatrogenic water intoxication in two cats. Journal of Veterinary Emergency & Critical Care23, 53–57.
- Lipschitz S., Campbell A.J., Roberts M.S., Wanwimolruk S., McQueen E.G., McQueen M. & Firth L.A. (1991) Subcutaneous fluid administration in elderly subjects: Validation of an under-used technique. Journal of the American Geriatrics Society39, 6–9.
- Muir W.W., Kijtawornrat A., Ueyama Y., Radecki S.V. & Hamlin R.L. (2011) Effect of intravenous administration of lactated Ringers solution on hematologic, serum biochemical, rheological, hemodynamic, and renal measurements in healthy isoflurane-anesthetized dogs. Journal of the American Veterinary Medical Association239, 630–637.
- Noriega O.D. & Blasco S.A. (2014) Efficacy of the subcutaneous route compared to intravenous hydration in the elderly hospitalised patient: a randomised controlled study. Revista española de geriatría y gerontología49, 103–107.
- O'Keeffe S.T. & Lavan J.N. (1996) Subcutaneous fluids in elderly hospital patients with cognitive impairment. Gerontology42, 36–39.
- Paydar S., Bazrafkan H., Golestani N., Roozbeh J., Akrami A. & Moradi A.M. (2014) Effects of intravenous fluid therapy on clinical biochemical parameters of trauma patients. Emergency2, 90–95.
- Polzin D.J., Osborne C.A. & Ross S. (2005) Chronic kidney disease. In: Textbook of Veterinary Internal Medicine. 6th edn, 1756–1785. (eds S.J. Ettinger & E.C. Feldman), Saunders: Philadelphia.
- Rohrig R. (2012) RonnT, Lendemans S, Feldkamp T, de Groot H, Petrat F. Adverse effects of resuscitation of lactated Ringer compared with Ringer solution after severe hemorrhagic shock in rats. Shock38, 137–145.
- Rohrig R., Wegewitz C., Lendemans S., Petrat F. & de Groot H. (2014) Superiority of acetate compared with lactate in a rodent model of severe hemorrhagic shock. Journal of Surgical Research186, 338–345.
- Rose R.J., Carter R.J. & Ilkiw J.E. (1980) Some physiological and biochemical effects of intravenous fluid administration during halothane anaesthesia in the dog. Journal of Veterinary Pharmacology and Therapeutics3, 111–119.
- Rosol T.J., Chew D.J., Hammer A.S., et al. (1994) Effect of mithramycin on hypercalcemia in dogs. Journal of the American Animal Hospital Association30, 244–250.
- Schaer M. (1989) General principles of fluid therapy in small animal medicine. Veterinary Clinics of North America Small Animal Practice19, 203–213.
- Schenck P.A., Chew D.J., Nagode L.A., et al. (2012) Disorders of calcium: hypercalcemia and hypocalcemia. In: Fluid, Electrolyte and Acid-Base Disorders in Small Animal Practice. 4th edn, 121–194. (ed S.P. DiBartola), Saunders: Philadelphia.
- Slesak G., Schnurle J.W., Kinzel E., Jakob J. & Dietz K. (2003) Comparison of subcutaneous and intravenous rehydration in geriatric patients: a randomized trial. Journal of the American Geriatrics Society51, 155–160.
- Tollofsrud S., Elgjo G.I., Prough D.S., Williams C.A., Traber D.L. & Kramer G.C. (2001) The dynamics of vascular volume and fluid shifts of lactated Ringers solution and hypertonic-saline-dextran solutions infused in normovolemic sheep. Anesthesia and Analgesia93, 823–831.
- Valverde A., Hatcher M.E. & Stampfli H.R. (2008) Effects of fluid therapy on total protein and its influence on calculated unmeasured anions in the anesthetized dog. Journal of Veterinary Emergency & Critical Care18, 480–487.
- Williams E.L., Hildebrand K.L., McCormick S.A. & Bedel M.J. (1999) The effect of intravenous lactated Ringers solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesthesia and Analgesia88, 999–1003.
- Wright B.D. & Hopkins A. (2008) Changes in colloid osmotic pressure as a function of anesthesia and surgery in the presence and absence of isotonic fluid administration in dogs. Veterinary Anaesthesia and Analgesia35, 282–288.
- Zontine W.J. & Donovan M.L. (1969) Effect of hypodermoclysis with dextrose in dogs. Journal of the American Veterinary Medical Association30, 605–609.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?