(Created page with " ==Abstract== The choice of a suitable pretreatment method and the adjustment of the pretreatment parameters for efficient conversion of biomass are crucial for a successful...") |
m (Scipediacontent moved page Draft Content 718558801 to Njoku et al 2013a) |
(No difference)
| |
Latest revision as of 14:10, 1 June 2017
Abstract
The choice of a suitable pretreatment method and the adjustment of the pretreatment parameters for efficient conversion of biomass are crucial for a successful biorefinery concept. In this study, cocksfoot grass, a suitable lignocellulosic biomass with a potential for large-scale production was investigated for cellulosic ethanol production. The biomass raw materials were pretreated using wet explosion (WEx) at 25% dry matter concentration with addition of oxygen or dilute sulfuric acid. The enzymatic hydrolysis of cellulose was significantly improved after pretreatment. The highest conversion into cellulose monomeric C6 sugars was achieved for WEx condition AC-E (180°C, 15 min, and 0.2% sulfuric acid). For that condition, the highest ethanol yield of 197 g/kg DM (97% of theoretical maximum value) was achieved for SSF process by Saccharomyces cerevisiae. However, the highest concentration of hemicellulose C5 sugars was found for WEx pretreatment condition O2-A (160°C, 15 min, and 6 bar O2) which means that the highest potential ethanol yield was found at this moderate pretreatment condition with oxygen added. Increasing the pretreatment temperature to 180–190°C with addition of oxygen or dilute sulfuric acid significantly degrades the solubilized hemicellulose sugars and thus, achieved the highest formation of by-products.
Introduction
Lignocellulosic biomass, such as cocksfoot grass and wheat straw, is an attractive feedstock that has potential to produce considerable amounts of ethanol as a promising alternative fuel for transportation sector. The ethanol produced from these biomass resources are clean renewable energy and thus, present a sustainable form of energy alternatives to fossil fuels [1]. The lignocellulosic biomass is the most abundant biomass on earth, and its sources range from trees to agricultural residues [2]. These lignocellulosic biomass resources are highly complex, mainly comprised of cellulose, hemicellulose, and lignin that are not directly accessible for microbial degradation [3]. Cellulose and hemicelluloses are both carbohydrates polymers built-up by long chains of sugar monomers, which can be fermented into ethanol after pretreatment and hydrolysis by microbial action [4].
Production of ethanol as a liquid fuel from lignocellulosic biomass faces significant technical challenges, such as a need for pretreatment [4]. As lignocellulosic biomass is only partially degradable in their native form, various techniques of mechanical, chemical, and biological means have been developed to disrupt the lignocellulose structure, and make it susceptible to enzymatic and microbial action [5], such as wet explosion (WEx) [6], wet oxidation [7], dilute acid hydrolysis [8], and steam explosion [9]. To achieve high overall ethanol yields, the pretreatment should maximize the downstream enzymatic hydrolysis, and be effective for treatment of biomass at high dry matter concentrations [10]. Wet oxidation and dilute acid as pretreatment methods have been successfully implemented in laboratory and pilot-scale plants for treating several lignocellulosic biomass resources for the production of ethanol and bio-based products [8, 11]. Martin et al. [12] reported approximately 94% conversion efficiency of cellulose to monomeric glucose after enzymatic hydrolysis of cellulose fraction of clover–ryegrass mixtures subjected to wet oxidation, showing effective removal of lignin and hemicellulose from cellulose. However, this study was done with 6% dry matter concentrations making the results of limited interest for industrial implementation. Taherzadeh and Karimi [13] further reported that almost 100% hemicellulose removal is possible by dilute acid pretreatment. This method does, however, still need some improvements to make it economically interesting for biorefinery processes as the concentration of hexose sugars coming from the pretreatment stream needs to be increased as well as the current need for detoxification [14]. Furthermore, the pretreatment process cost should be reduced by operating at high dry matter concentrations, which will increase the final ethanol concentrations and, at the same time, reduce the reactor volume and minimize wastewater generation [10].
During pretreatment, lignocellulosic biomass is converted from its native form, in which it is highly recalcitrant to cellulose enzyme systems, into a form for which cellulose hydrolysis is effective [15]. The main purpose of pretreatment is to break the lignocellulosic structure [16], and to reduce the cellulose crystallinity with low loss of sugar compounds [17, 18]. At the same time, the formation of degradation products that inhibit the microbial activities during ethanol fermentation should be kept low [19].
In this study, WEx was applied to cocksfoot grass at high dry matter concentration w/w of 25%. The current study is based on previous investigations on WEx with addition of dilute sulfuric acid at a lower dry matter concentration w/w of 14% [20]. The effects of different combinations of WEx pretreatment parameters on the biomass composition and enzymatic hydrolysis of the treated substrate was evaluated. Subsequently, the conversion of hexose sugars of cocksfoot grass into ethanol by Saccharomyces cerevisiae was investigated.
Material and Methods
Raw material
Cocksfoot grass from the island of Bornholm, Denmark, was harvested in August. The biomass sample was air-dried and hammer milled to a particle size of 2–3 mm and stored in plastic bags at room temperature prior to pretreatment. A portion of the raw biomass was ground in a coffee grinder (Butler UGS, Zhejiang, China) to pass a 1-mm screen and used for chemical composition analysis. Dry matter contents (DM), volatile solid contents (VS), and ash were determined according to the procedure described by the American Public Health Association [21].
The content of total carbohydrates (cellulose and hemicellulose), and Klason lignin in the raw biomass was determined by strong acid hydrolysis according to the procedure developed by the National Renewable Energy Laboratory [22]. Subsequently, sugar analysis (glucose and xylose) was performed by high-performance liquid chromatography (HPLC) refractive index (RI) equipped with an Aminex HPX-87P column (Bio-Rad Laboratories, Hercules, CA) at 83°C with deionized water (Thermo Scientific, Barnstead Nanopure, Dubuque, IA) as an eluent with a flow rate of 1.0 mL/min. Prior to HPLC analysis, samples were centrifuged at 4000 g for 10 min, and filtered through a 0.45 μm syringe filter.
The VS content of the raw biomass found as the difference between the total VS value and the sum of the carbohydrate fractions analyzed in the raw biomass was referred to as “other organic matter,” which is the sum of proteins, fats, and volatile compounds.
WEx pretreatment
The WEx pretreatment was performed batchwise by suspending the raw cocksfoot grass with tap water to reach a dry matter concentration w/w of 25% in a 10-L high-pressure reactor constructed at the Center for Bioproducts and Bioenergy, Washington State University, Richland, WA, USA [6]. The reactor was equipped with a gas/liquid inlet for injection of dilute sulfuric acid or oxygen pressure, and a continuous stirrer (2000 r.p.m.). The reactor was heated by a water jacket connected to a heat exchanger controlled by an oil heater. The temperature and pressure inside the reactor were monitored by two temperature sensors and one pressure sensor both mounted in the headspace and in the bottom of the reactor. The pretreatment was carried out at different temperatures based on the previous optimization trials with the following conditions: temperature (160–190°C), oxygen pressure O2 (6 bars), and dilute sulfuric acid concentration (0.2%) at a retention time of (15 min). The conditions of pretreatment trials are presented in Table 1. The acid concentration or oxygen pressure was injected into the pretreatment reactor after the desired temperature was reached. After the treatment, the biomass was flashed into a 100-L flash tank connected to the reactor, resulting in a sudden drop in temperature and pressure.
| Condition | Temperature (°C) | R/T (min) | Oxygen (bar) | Acid concentration (%) |
|---|---|---|---|---|
| ||||
| O2-A | 160 | 15 | 6 | – |
| O2-B | 170 | 15 | 6 | – |
| O2-C | 180 | 15 | 6 | – |
| AC-D | 170 | 15 | – | 0.2 |
| AC-E | 180 | 15 | – | 0.2 |
| AC-F | 190 | 15 | – | 0.2 |
The resulting biomass slurry from the pretreatment was separated into liquid and solid fractions by vacuum filtration. The solid fraction was washed thoroughly with milliQ water and stored in a freezer (−16°C) prior to compositional analysis and further processing. The separated liquid fraction was stored at 5°C for further analyses.
Analysis of the solid and liquid fraction
The washed solid fraction (portion) obtained after separation of the WEx slurry was dried in an incubator at 38°C for 12 h before compositional analysis. The dried solids were ground in a coffee grinder (Butler UGS) to pass a 1-mm screen before chemical compositional analysis. The content of total carbohydrates (cellulose and hemicelluloses), and Klason lignin in the separated solid fractions was determined by strong acid hydrolysis as previously described [22].
Carbohydrates in the liquid fractions (filtrate) after WEx pretreatment were both polymers and oligomers together with small amounts of monomers, and hence the samples were hydrolyzed using 4% (w/w) sulfuric acid at 121°C for 10 min to determine the total xylose, arabinose, and glucose concentration in the filtrate. The analysis was determined according to the National Renewable Energy Laboratory protocol [23]. Glucose, xylose, arabinose present in the liquid fractions were quantified by HPLC as described in Section “'Raw material'”, and furfural, hydroxymethylfurfural (HMF), and acetic acid also present in the liquid fractions were measured by HPLC RI equipped with an Aminex HPX-87H column (Bio-Rad Laboratories) at 60°C with 4 mmol/L H2SO4 as an eluent with a flow rate of 0.6 mL/min.
Enzymatic convertibility of cellulose in the solid fraction
The release of hydrolysable cellulose by WEx pretreatment was analyzed by the sugars released after enzymatic hydrolysis using a commercial enzyme mixture (Cellic CTec2), kindly provided by Novozymes North America (Franklinton, NC). The enzymatic convertibility of the separated WEx solid fraction was carried out at 10% DM with 0.05 mol/L succinate buffer (pH 5.0). The experiments were performed in duplicates in 2 mL Eppendorf tubes filled with 1.5 mL of hydrolysis media and an enzyme dosage of 20 mg-EP/g-VS (EP, enzyme protein) for all samples. The hydrolysis mixture was incubated for 72 h at 50°C in a thermomixer shaker at 1400 r.p.m. The reaction was stopped by heating the solution to 100°C for 10 min, mixed by vortexing, and centrifuged for 8 min at 3600 g. The concentration of glucose, xylose, and arabinose in the hydrolyzate was quantified by HPLC as previously described in Section “'Raw material'”.
Ethanol fermentation process
The hexose sugars from the WEx separated solid fraction were fermented to ethanol through simultaneous saccharification and fermentation (SSF) by S. cerevisiae (Thermosacc®, Lafayette, CO). The S. cerevisiae inoculum culture medium was prepared aseptically in 250-mL shaking flask covered with cotton stopper with 100 mL medium containing 10 g/L yeast extract, 20 g/L peptone, and 20 g/L d-glucose, and incubated on a rotary shaker at 160 r.p.m. and 32°C for 24 h. All media were sterilized by autoclaving at 121°C for 30 min. The cells were harvested by centrifugation, and the pellet was collected for SSF fermentation to a final optical density (OD) of 0.5 measured at (600 nm), corresponding to a cell concentration of around 0.9 g/L. Presaccharification (liquefaction) and SSF were performed under anaerobic condition in sterile 250 mL shaking flasks with 100 mL fermentation media as the working volume.
Presaccharification of the material was performed at 50°C for 6 h at an enzyme dosage of 10 mg-EP/g-VS using Cellic CTec2, adjusted to pH 4.8 with 1 mol/L citrate buffer to liquefy the solid fractions and ensure a proper substrates mixing. After presaccharification, the shaking flasks were then cooled to room temperature and supplemented with second batch of enzyme mixture (Cellic CTec2) at an enzyme dosage of 10 mg-EP/g-VS, inoculated with the yeast cells under aseptic condition and the pH was maintained at 4.8 by addition of 0.05 mol/L citrate buffer solution. The shaking flasks were sealed with bubbler airlocks filled with water and incubated on a rotary shaker at 150 r.p.m. and 32°C for 168 h. Samples were withdrawn at regular intervals for sugar and ethanol concentrations and were determined by HPLC as described previously in Section 'Raw material'. All the experiments were performed in duplicates at the same initial cell concentration.
Calculations
The recovery of sugars in the solid or filtrate during the WEx pretreatment process was calculated according to Eq. (1). The yields after WEx and enzymatic hydrolysis of the solid fractions were calculated according to Eq. (2) for glucose released and Eq. (3) for pentoses released (xylose and arabinose): where glucoseEH is the mass of glucose released after enzymatic hydrolysis of cellulose in the solid fraction and pentosesLF is the mass of pentose sugars (xylose and arabinose) released after WEx pretreatment in the liquid fraction. The ethanol yield (YEtOH) was calculated by dividing the total amount of ethanol produced by the initial dry weight of treated cocksfoot grass. The percent theoretical (stoichiometric) ethanol yield (% YEtOH) was calculated according to Eq. (4): where 1.11 is the stoichiometric conversion factor of cellulose to equivalent glucose and 0.51 is the theoretical ethanol yield (in grams) generated per 1 g of glucose [24]. This yield is always <100% as part of the sugars is converted to cell mass and by-products by the organisms.
|
|
(1) |
|
|
(2) |
|
|
(3) |
|
|
(4) |
Results and Discussion
Raw material composition
The chemical composition of raw material shows that it has a high concentration of cellulose and contains a fair amount of hemicelluloses (Table 2), which is comparable or higher than the amounts found in wheat straw (33.9 g and 23.0 g/100 g DM for cellulose and hemicellulose, respectively) [25]. The DM is approximately 93%, which makes it attractive as ethanol feedstock. The lignin content is relatively higher than the content of other organic matter, which is the sum of nonanalyzed organic matter like pentoses other than xylose and arabinose, proteins and fats in biomass.
| g DM/100 g biomass | Cocksfoot grass 92.9 |
|---|---|
| Composition biomass [g/100 g DM]a | |
| |
| Cellulose | 35.73 (0.31) |
| Hemicelluloses | 23.71 (0.06) |
| Klason lignin | 18.74 (0.04) |
| Ash | 5.23 (0.04) |
| Other organic matter | 16.60 (0.00) |
WEx pretreatment and recovery of carbohydrates and lignin
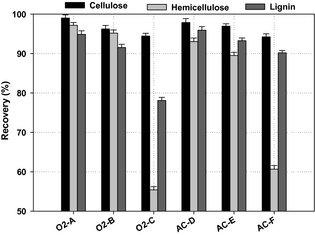
As shown in (Fig. 1), the recovery of carbohydrates in the solid and liquid fractions (cellulose and hemicellulose) and lignin found in the solid fraction varied with the conditions of the WEx pretreatment process. The WEx pretreatment was expected to fractionate the lignocellulosic material into a solid fraction containing mainly cellulose and lignin, and a liquid fraction enriched with solubilized hemicelluloses mainly present as C5 monomers, and low-molecular lignin fragments in dissolved form.
|
|
|
Figure 1. Recovery of polysaccharides (cellulose and hemicellulose) and lignin after wet explosion pretreatment at different conditions (error bars represent standard deviations of duplicate measurements). |
The most efficient cellulose and hemicelluloses recovery of 99% and 97%, respectively, was found for WEx pretreatment condition O2-A (160°C, 15 min, 6 bar O2), higher than previously reported for high dry matter concentrations. The lowest recovery of cellulose at 94% was found for WEx pretreatment conditions AC-F and O2-C (190°C, 15 min, 0.2% sulfuric acid and 180°C, 15 min and 6 bar O2). The same conditions (O2-C and AC-F) achieved the lowest hemicelluloses recovery at 55% and 61%, respectively (Fig. 1). Generally, we found high recovery of cellulose and hemicelluloses under WEx pretreatment at temperature below 180°C, whereas, applying the process temperature above 170°C significantly degraded significant parts of the hemicellulose sugars, especially, WEx pretreatment temperature at 180°C and 6 bars O2 resulting in loss of 45% of the hemicellulose sugars and thus, gave the lowest recovery of the hemicellulose fractions. The recovery of cellulose and hemicelluloses found for WEx pretreatment with the lowest severe condition (O2-A) is higher than what has been previously reported with lignocellulosic biomass subjected to various pretreatment methods. Petersson et al. [4] achieved 92% and 69% recovery of cellulose and hemicellulose sugars from faba bean straw under wet oxidation pretreatment at 195°C for 15 min, 2 g/L Na2CO3, and 12 bar O2. WEx pretreatment condition (AC-D) achieved cellulose and hemicellulose recovery of 97% and 93%, respectively; which is higher than the recovery of 93% and 72% for cellulose and hemicellulose, respectively, reported by Georgieva et al. [10] after subjecting wheat straw to WEx pretreatment at 180°C, 35% (v/v) hydrogen peroxide for 15 min. Operational parameters for pretreatment must be tailored to the specific biomass structural composition. It was evident in this study that the combination of pretreatment temperature, dilute acid, and/or oxygen pressure was the most crucial factor for gaining high recovery of sugars for cocksfoot grass biomass.
The high recovery of cellulose among the tested pretreatment conditions reveals an efficient separation of the cellulose from lignin and low degradation of cellulose to other products, such as HMF during the WEx pretreatment process. On the other hand, hemicellulose recovery varied in a wider range among the pretreatment conditions than cellulose, and the conditions with higher pretreatment severity significantly degraded hemicellulose sugars, and accordingly achieved below 90% hemicellulose recovery.
The highest degree of lignin solubilization was achieved in pretreatment condition O2-C, due to the combination of high pretreatment temperature and oxygen pressure as an oxidizing agent. This is in agreement with the fact that lignin tends to undergo both cleavage and oxidation during wet oxidation which facilitates the solubilization of lignin during pretreatment with oxidizing agent [13]. Generally, the lignin recovery in the solid fraction among the pretreatment conditions was above 70% (Fig. 1). The highest recovery of lignin in the solid fraction was obtained under WEx pretreatment condition AC-D showing approximately 96% lignin recoveries. As lignin recovery in the solid fraction after pretreatment is affected by solubilization and depolymerization of sugars [26], lignin may be degraded to low-molecular lignin compounds, that is, phenolic compounds under harsh pretreatment conditions [27]. The high recovery of lignin in the solid fraction indicates that only a small part of lignin was solubilized under the WEx conditions with low pretreatment temperatures and, hence, the formation of low-molecular lignin compounds was low.
Enzymatic hydrolysis of cellulose in the solid fraction and yield of sugars in the liquid fraction after pretreatment
The enzymatic accessibility of the cellulose was investigated for all the pretreated solid fractions under WEx conditions as this is one of the most important factors to evaluate the efficiency of the pretreatment for the production of ethanol from hexose sugars [28].
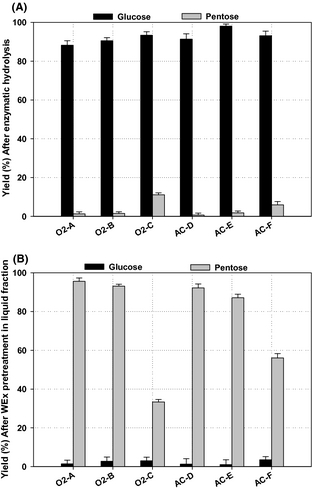
Enzymatic convertibility of cellulose in solid fraction pretreated at high dry matter concentration (25%) varied between 88% and 98% for the different pretreatment conditions (Fig. 2A). The highest convertibility of cellulose in the solid fraction, 98%, was achieved for the WEx condition AC-E (180°C, 15 min, 0.2% sulfuric acid), and this is in accordance with that dilute acid pretreatment is not effective in lignin solubilization, but it can disrupt lignin and increase the cellulose susceptibility to enzymatic action [13]. However, good results were also achieved for WEx pretreatment conditions O2-C and AC-F (180°C, 15 min, 6 bar O2 and 190°C, 15 min, 0.2% sulfuric acid) resulting in a 93% glucose yield. The lowest glucose yield was obtained at WEx condition O2-A, although this condition gave the highest overall cellulose and hemicellulose recovery. This result supports the idea that the optimum conditions for the highest sugar recovery do not necessarily mean the most effective conditions for enzymatic conversion of cellulose fractions to sugar monomers [13]. It has been previously reported that combining high pretreatment temperature and prolonging the treatment time could increase the efficiency of hydrolysis of cellulose materials [29], and a high pretreatment temperature is more suitable for achieving high glucose yields [30]. In comparison, Martin et al. [12] reported 93.6% cellulose conversion of the solid fraction after wet oxidation pretreatment of clover–ryegrass mixtures at 195°C, 10 min, and 1.2 MPa. It has been previously demonstrated that pretreatment of lignocellulosic biomass at high dry matter concentration is an ideal method to improve ethanol yields; Lu et al. [31] found 63.7% glucose yield after enzymatic hydrolysis of pretreated rapeseed straw under sulfuric acid-catalyzed hydrothermal pretreatment at 180°C, 1% sulfuric acid, 20% solids content for 10 min. Generally, all the pretreatment conditions tested in our study achieved good glucose yields above 88%, which is higher than previously reported for biomass pretreatment at high dry matter concentrations as used in our study.
|
|
|
Figure 2. Sugar yields after wet explosion (WEx) pretreatment and enzymatic hydrolysis: (A) Yield of glucose and pentoses after enzymatic hydrolysis of solid fractions; and (B) Yield of glucose and pentoses in liquid fractions after WEx pretreatment. Average values and standard deviation reported for duplicates analysis. |
The pentose sugars yield (xylose and arabinose) are presented in (Fig. 2B). The highest solubilized pentoses was found under the less severe conditions; pretreatment condition O2-A (160°C, 15 min, 6 bar O2) gave approximately 96% yield of pentose sugars. Furthermore, pretreatment conditions O2-B and AC-D (170°C, 15 min, 6 bar O2 and 170°C, 15 min, 0.2% sulfuric acid) achieved a yield of 93% and 92%, respectively, which is higher than 68% total pentoses yield achieved by Thomsen et al. [25] for wet oxidation pretreatment of wheat straw at 190°C, H2O2 and 6 min. The pretreatment conditions (O2-C and AC-F) significantly degraded a large fraction of the pentose sugars and thus, achieved the lowest yield of around 33% and 56%, respectively, showing that lower pretreatment temperature is more advantageous for maximizing pentose yields. The extraction of hemicellulose sugars in the liquid fraction was more pronounced in lower pretreatment severity, especially, the pretreatment at temperature of 160°C. It is obvious that the release of hexose and pentose sugars needs different pretreatment severity, as the conditions where the best hexose sugars were obtained will convert free pentose sugars found in the solution.
The total yield of sugars (hexose and pentose) is presented in Table 3. This was determined based on the total cellulose and hemicellulose sugars found in the solid and liquid fractions after pretreatment and enzymatic treatment. Total yield of sugars was 526.3–542.0 g/kg DM, corresponding to 88–91% of theoretical in most of the WEx pretreatment conditions, but was lower (475 g/kg DM, corresponding to 80% of total yield of sugars) for the most severe tested pretreatment conditions O2-C and AC-F (180°C, 15 min, 6 bar O2 and 190°C, 15 min, 0.2% sulfuric acid) owing to the high degree of sugar degradation to other products such as HMF and furfural in these conditions. For an economically viable process, the best condition was identified as O2-B with respect to low formation of by-products and yield of both C5 and C6 sugars. Considering the overall process economy with regard to process temperature (energy consumption), condition O2-A can be identified as the best WEx pretreatment condition for achieving both high total yield of sugars (526 g/kg DM, which amounts to 89%) with moderate energy input. Pretreatment conditions AC-D, O2-B, and AC-E gave slightly higher total sugars yields (1–2%; 528, 533, and 542 g/kg DM, respectively) as shown in Table 3, but here the temperature was increased by 10 and 20°C, respectively. It is therefore, crucial to consider all parameters before the pretreatment conditions are selected. Pretreatment reactors for dilute acid pretreatment will need to be made of acid-resistant materials which will add extra cost compared to reactors operated with oxygen addition.
| WEx conditions | Total Yield of Sugars | |
|---|---|---|
| g/kg DM | % | |
| ||
| O2-A | 526.3 ± 0.14 | 88.54 |
| O2-B | 533.2 ± 0.17 | 89.70 |
| O2-C | 474.7 ± 0.11 | 79.86 |
| AC-D | 528.3 ± 0.08 | 88.88 |
| AC-E | 542.0 ± 0.15 | 91.18 |
| AC-F | 475.7 ± 0.06 | 80.03 |
Formation of degradation products
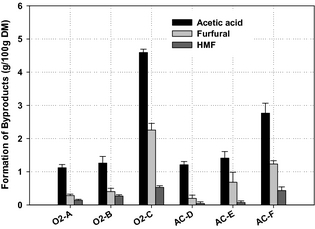
The formation of acetic acid was higher among the WEx pretreatment conditions (Fig. 3), as this was the main degradation products measured in the liquid fraction of the pretreated material. The highest formation of acetic acid (4.59 g/100 g DM) was found for pretreatment condition O2-C, followed by pretreatment condition AC-F, with a level of 2.77 g/100 g DM. The formation of acetic acid during pretreatment has been reported elsewhere in the literature to be associated with high pretreatment temperature and longer treatment time [11]. This is evident as the pretreatment conditions (O2-C and AC-F) for oxygen and dilute sulfuric acid, respectively, gave the highest formation of acetic acid, which is further confirmed by the lower yield of hemicellulose sugars associated with the above-mentioned conditions. Depending on the process severity, carboxylic acids (mainly acetic acid), furan derivatives (furfural and 5-hydroxymethyl furfural-HMF), and phenolic compounds are generated during pretreatment of lignocellulosic biomass, and are considered potential fermentation inhibitors [32, 33]. At more severe pretreatment conditions, hemicellulose sugar monomers are degraded to furfural while HMF is formed from hexose degradation, and phenolic compounds are liberated from partial breakdown of lignin [34].
|
|
|
Figure 3. Formation of by-products in the liquid fractions after wet explosion pretreatment. Results are average of duplicates and error bars represent standard deviations. |
Furfural and HMF formation was low in most of the pretreatment conditions. The highest concentration of furfural was found under condition (O2-C), around 2 g/100 g DM (Fig. 3). HMF formation was the lowest among the two by-products. The formation of furfural and HMF in the liquid fraction during pretreatment is a result of the dehydration of pentose and hexose sugars, respectively, under thermal and acidic conditions [35]. The results presented in (Fig. 3) show that the most severe pretreatment conditions achieved the highest concentrations of the measured by-products in the WEx liquid fraction. This is in agreement with previous investigations where the production of these compounds increases with higher pretreatment temperatures [19], and these compounds not only reduce the sugar yield but can also inhibit the fermentation process. The formation of the by-products found in this study is in good agreement with Martin et al. [35], who reported relatively the same amounts of these by-products for sugarcane bagasse subjected to wet oxidation with pretreatment conditions (195°C, 15 min, and alkaline pH) gave 9.21 g/100 g material for carboxylic acids (acetic acid and glycolic acid), 0.53 g/100 g material for furfural, and 0.07 g/100 g material for HMF, respectively, compared to our highest found values of (g/100 g DM): acetic acid, 4.59; furfural, 2.26; and HMF, 0.53.
Simultaneous saccharification and fermentation
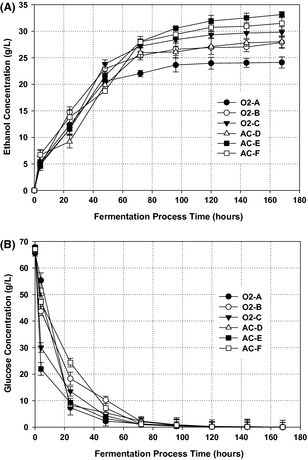
The separated solid fractions enriched in cellulosic sugars after pretreatment were subjected to an SSF process with simultaneous hydrolysis and fermentation to ethanol using S. cerevisiae. Prior to the SSF process, the substrate was liquefied for 6 h enabling sufficient mass transfer in the fermentation broth and, thus decrease the viscosity of the material inside the fermentation medium. The yeast S. cerevisiae exhibit high tolerance toward fermentation inhibitors and is the most efficient organism for glucose fermentation [10]. The ethanol and glucose concentration profiles during the fermentation are presented in (Fig. 4A and B), as the concentration of glucose decreased to nearly 2 g/L, the ethanol concentrations rapidly increased from 0 to around 27 g/L during the first 72 h of fermentation (Fig. 4A). This is comparable with what has been reported elsewhere in the literature [36]. After 72 h, glucose was still released from the cellulose fraction at a very low rate (Fig. 4B); this was observed by a slight increase in ethanol concentration which was kept stable after 120 h. A lag phase was not observed during the course of fermentation, probably due to subsequent washing of the solid fractions prior to SSF process, thereby reducing the risk of containing fermentation inhibitors at inhibitory level.
|
|
|
Figure 4. Ethanol production profile: (A) Time course of ethanol formation during simultaneous saccharification and fermentation (SSF) process of cellulose fraction by Saccharomyces cerevisiae incubated over 168 h, 150 r.p.m. at 32°C, and pH 4.8; and (B) Time course of glucose utilization during ethanol fermentation over 168 h. Values are means of duplicate experiments. |
An ethanol concentration of about 33.14 g/L was achieved under the pretreatment condition AC-E, corresponding to a yield of 197.3 g/kg DM (97% of the theoretical maximum yield), showing that under this condition all the C6 sugars present in the solid fraction can be converted to sugar monomers and simultaneously fermented to ethanol. This is the highest achieved ethanol yield in our study for C6 conversion by S. cerevisiae (Table 4). Surprisingly, condition AC-F (190°C, 15 min, 0.2% sulfuric acid) achieved ethanol yield of 187.2 g/kg DM (92% of the theoretical maximum value) lower than condition AC-E (180°C, 15 min, 0.2% sulfuric acid), showing that this pretreatment condition has partially degraded some of the cellulose sugars, presumably due to the severe destruction of the cellulose crystalline structure. On the other hand, condition O2-C (180°C, 15 min, 6 bar O2) also gave a lower ethanol yield compared to condition (AC-E) at the same pretreatment temperature. It has been previously documented that pretreatment at elevated temperature combined with oxygen pressure can partly degrade cellulose fractions to other by-products due to oxidation reaction that associated with oxygen [13]. The 97% yield of the theoretical maximum value found in this study is in good agreement with the previous study on SSF process with S. cerevisiae [25].
| WEx conditions | Final ethanol concentration (g/L) | Ethanol yield (g/kg DM) | % of theoretical Yield |
|---|---|---|---|
| |||
| O2-A | 24.12 ± 0.05 | 141.81 ± 0.09 | 69.9 |
| O2-B | 27.89 ± 0.07 | 158.59 ± 0.03 | 78.2 |
| O2-C | 29.80 ± 0.02 | 177.95 ± 0.13 | 87.7 |
| AC-D | 28.02 ± 0.04 | 166.75 ± 0.16 | 82.2 |
| AC-E | 33.14 ± 0.17 | 197.34 ± 0.06 | 97.3 |
| AC-F | 31.49 ± 0.14 | 187.19 ± 0.04 | 92.3 |
The lowest achieved ethanol yield of 141.8 g/kg DM (70% of the theoretical maximum) was found under the less severe condition (O2-A). The reason could be that the cellulose structure had not been significantly altered under this condition and, hence, could not easily be hydrolyzed to cellulose for simultaneous conversion into ethanol by S. cerevisiae. However, good results were also obtained from other conditions (O2-B and AC-D), around 80% of the theoretical maximum possible yield. This result is higher or comparable to ethanol yields reported for wheat straw and clover–ryegrass mixtures solid fractions fermented to ethanol by S. cerevisiae [12, 27].
Conclusions
Our present investigation on cocksfoot grass revealed that WEx is a promising pretreatment method for producing high sugar and ethanol yields. The highest monomeric C6 sugars release from the cellulose fraction after WEx pretreatment and enzymatic hydrolysis was attributed to condition AC-E (180°C, 15 min, 0.2% sulfuric acid), on the other hand, the release of hemicellulose sugars in the liquid fraction was more pronounced under WEx condition O2-A (160°C, 15 min, 6 bar O2) where a significant higher yield was achieved. From these results, we found that disrupting the biomass complex structures using oxygen pressure combined with temperatures around 180°C results in destruction of both hexose and pentose sugars, where significant amount of sugars was degraded to by-products such as acetic acids. This is evident as the main reactions for oxidative pretreatment at high temperatures are the formation of acids. The WEx pretreatment condition (AC-E) at 180°C achieved the highest ethanol yield of 197 g/kg DM (97% of theoretical) from the cellulose fraction subjected to SSF process, whereas, the WEx condition (O2-C) at 180°C gave slightly lower ethanol yield of 188 g/kg DM. It is quite obvious from this study that the release of hexose and pentose sugars have very different dynamics and, therefore, pretreatment process parameters should be tailored to the specific biomass compositional structures and with a view to all the potential sugars which can be produced.
Acknowledgments
This work is financially supported by the Energy Technology Development and Demonstration Programme of the Danish Energy Council, grant no.: 64009-0010.
Conflict of Interest
None declared.
References
- Nutawan, Y., P. Phattayawadee, T. Pattranit, and N. E. Mohammad. 2010. Bioethanol produced from rice straw. Energy Res. J.1:26–31.
- Agbogbo, F. K., and K. S. Wenger. 2007. Production of ethanol from corn stover hemicellulose hydrolyzate using Pichia stipitis . J. Ind. Microbiol. Biotechnol.34:723–727.
- Chen, H., and W. Qiu. 2010. Key technologies for bioethanol production from lignocellulose. Biotechnol. Adv.28:556–562.
- Petersson, A., M. H. Thomsen, H. Hauggaard-Nielsen, and A. B. Thomsen. 2007. Potential bioethanol and biogas production using lignocellulosic biomass from winter rye, oilseed rape and faba bean. Biomass Bioenergy31:812–819.
- Xuejun, P., and S. Yoshihiro. 2005. Fractionation of wheat straw by atmospheric acetic acid process. Bioresour. Technol.96:1256–1263.
- Rana, D., V. Rana, and B. K. Ahring. 2012. Producing high sugar concentrations from loblolly pine using wet explosion pretreatment. Bioresour. Technol.121:61–67.
- Schmidt, A. S., and A. B. Thomsen. 1998. Optimization of wet oxidation pretreatment of wheat straw. Bioresour. Technol.64:139–151.
- Saha, B. C., L. B. Iten, M. A. Cotta, and Y. V. Wu. 2005. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem.40:3693–3700.
- Ballesteros, I., M. J. Negro, J. M. Oliva, A. Cabanas, and M. Ballesteros. 2006. Ethanol produced from steam-explosion pretreated wheat straw. Appl. Biochem. Biotechnol.129–132:496–508.
- Georgieva, T. I., X. Hou, T. Hilstrøm, and B. K. Ahring. 2008. Enzymatic hydrolysis and ethanol fermentation of high dry matter Wet-Exploded wheat straw at low enzyme loading. Appl. Biochem. Biotechnol.148:35–44.
- McGinnis, G. D., W. W. Wilson, and C. E. Mullen. 1983. Biomass pretreatment with water and high-pressure oxygen. The wet-oxidation process. Industrial Engineering Chemistry Product Research. Development22:352–357.
- Martin, C., M. H. Thomsen, H. Hauggaard-Nielsen, and A. B. Thomsen. 2008. Wet oxidation pretreatment, enzymatic hydrolysis and simultaneous saccharification and fermentation of clover-ryegrass mixtures. Bioresour. Technol.99:8777–8782.
- Taherzadeh, M. J., and K. Karimi. 2008. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci.9:1621–1651.
- Kuhad, R. C., R. Gupta, Y. P. Khasa, and A. Singh. 2010. Bioethanol production from lantana camara (red sage): pretreatment, saccharification and fermentation. Bioresour. Technol.101:8348–8354.
- Hendriks, A. T. W. M., and G. Zeeman. 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol.100:10–18.
- Kumar, P., D. M. Barrett, M. J. Delwiche, and P. Stroeve. 2009. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res.48:3713–3729.
- Karimi, K., G. Emtiazi, and M. J. Taherzadeh. 2006. Ethanol production from dilute–acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae . Enzyme Microb. Technol.40:138–144.
- Zheng, Y., Z. Pan, and R. Zhang. 2009. Overview of biomass pretreatment for cellulosic ethanol. Int. J. Agric. Biol. Eng.2:51–68.
- Mosier, N., C. Wyman, B. E. Dale, R. Elander, Y. Y. Lee, M. Holtzapple, et al. 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol.96:673–686.
- Njoku, S. I., H. Uellendahl, and B. K. Ahring. 2013. Tailoring wet explosion process parameters for the pretreatment of Cocksfoot grass for high sugar and ethanol yields. Manuscript submitted for publication.
- Greenberg, A. E., L. S. Clesceri, and R. R. Trussel. 1992. Standard methods for the examination of water and waste water. 18th ed. American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WAE), Washington, DC.
- Sluiter, A.B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton, et al. 2008. Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure, Technical Report NREL/TP-510-42618.
- Sluiter, A., B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, and D. Templeton. 2008. Determination of sugars, byproducts and degradation products in liquid fraction process samples. Laboratory Analytical Procedure, Technical Report NREL/TP-510-42623.
- Hatzis, C., C. Riley, and G. P. Philippidis. 1996. Detailed material balance and ethanol yield calculations for biomass-to-ethanol conversion process. Appl. Biochem. Biotechnol.57/58:443–459.
- Thomsen, M. H., A. Thygesen, H. Jørgensen, J. Larsen, B. H. Christensen, and A. B. Thomsen. 2006. Preliminary results on optimization of pilot scale pretreatment of wheat straw used in coproduction of bioethanol and electricity. Appl. Biochem. Biotechnol.129–132:448–460.
- Sassner, P., C. G. Mårtensson, M. Galbe, and G. Zacchi. 2008. Steam pretreatment of H2SO4–impregnated Salix for the production of bioethanol. Bioresour. Technol.99:137–145.
- Petersen, M., J. Larsen, and M. H. Thomsen. 2009. Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenergy33:834–840.
- Varga, E., A. S. Schmidt, K. Reczey, and A. B. Thomsen. 2003. Pretreatment of corn stover using Wet Oxidation to enhance enzymatic digestibility. Appl. Biochem. Biotechnol.104:0273–2289.
- Taherzadeh, M. J., and C. Niklasson. 2004. Ethanol from lignocellulosic materials: pretreatment, acid and enzymatic hydrolysis, and fermentation. ACS Symp. Ser.889:49–68.
- Sørensen, A., P. J. Teller, T. Hilstrøm, and B. K. Ahring. 2008. Hydrolysis of miscanthus for bioethanol production using dilute acid presoaking combined with wet explosion pre-treatment and enzymatic treatment. Bioresour. Technol.99:6602–6607.
- Lu, X., Y. Zhang, and I. Angelidaki. 2009. Optimization of H2SO4-catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: focusing on pretreatment at high solids content. Bioresour. Technol.100:3048–3053.
- Klinke, H. B., A. B. Thomsen, and B. K. Ahring. 2004. Inhibition of ethanol–producing yeast and bacteria by degradation products produced during pre–treatment of biomass. Appl. Microbiol. Biotechnol.66:10–26.
- Saha, B. C.2004. Lignocellulose biodegradation and applications in biotechnology. ACS Symp. Ser.889:2–34.
- Palmqvist, E., and B. Hahn-Hägerdal. 2000. Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresour. Technol.74:25–33.
- Martin, C., H. B. Klinke, and A. B. Thomsen. 2007. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb. Technol.40:426–432.
- Bertilsson, M., K. Olofesson, and G. Liden. 2009. Prefermentation improves xylose utilization in simultaneous saccharification and fermentation of pretreated spruce. Biotechnol. Biofuels2:1754–6834.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?