(Created page with " ==Abstract== Investigations into direct methane to methanol conversion are justified based on the avoidance of synthesis gas generation, which accounts for around 60% of th...") |
m (Scipediacontent moved page Draft Content 601741661 to Arno-de-Klerk 2014a) |
(No difference)
| |
Latest revision as of 14:10, 1 June 2017
Abstract
Investigations into direct methane to methanol conversion are justified based on the avoidance of synthesis gas generation, which accounts for around 60% of the capital cost of synthesis gas to methanol conversion. A significant body of information already exists on the process chemistry, but little has been reported on the engineering of such a process. An engineering evaluation of the process was performed and the potential of this process as a platform technology for small-scale gas-to-liquids (GTL) applications was evaluated. It was found that direct methane to methanol conversion had 35% carbon efficiency and 28% thermal efficiency, which were about half of the process efficiencies of indirect methanol synthesis using synthesis gas. The poor process efficiency was mainly a consequence of the irreversible loss of carbon to CO x during conversion. The direct methane to methanol process also required an air separation unit, which eroded the stated benefit of avoiding a synthesis gas generation step in the process. The utility footprint was typical of GTL processes, with large gas compression duties and cooling duties. Overall, the engineering evaluation indicated there was no benefit to employ direct methane to methanol conversion instead of indirect methanol synthesis (the industry standard), and there was no specific benefit of direct methane to methanol conversion, irrespective of scale, for GTL applications.
Introduction
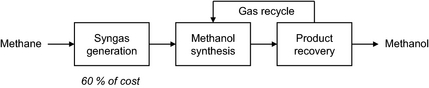
Methanol is a large volume commodity chemical. Industrially methanol is produced mainly by indirect liquefaction of natural gas [1]. The indirect liquefaction process involves three steps (Fig. 1). The first step is reforming of natural gas to synthesis gas, which is then in the second step converted into methanol. Synthesis gas conversion to methanol is conducted over a Cu-ZnO-based catalyst and methanol is obtained with high selectivity. The conversion of synthesis gas to methanol is equilibrium limited [2]. In the third step, the crude methanol is recovered from the unconverted synthesis gas and purified, while the unconverted synthesis gas is recycled. The most expensive step in process is synthesis gas generation, which accounts for 60% of the capital cost of the process [3].
|
|
|
Figure 1. Simplified block flow diagram of indirect methanol synthesis. |
Methanol can also be produced by the direct partial oxidation of methane to methanol. There is a considerable body of literature on the process chemistry of direct methane to methanol conversion [4]. It is one of a class of processes that involves the reaction of methane with an oxidant and direct oxidative methane conversion is often discussed collectively [[[#ese351-bib-0005|[5]]], [6], [7], [8]]. Studies that consider the engineering aspects of processes for direct oxidative methane conversion are less abundant [[[#ese351-bib-0009|[9]]], [10]].
On paper the direct conversion of methane to methanol seems like a good idea. The trade-off between conversion and product selectivity is anticipated and can in principle be overcome by the recycling of unconverted methane. Examples of industrial processes for the direct oxidation of methane to methanal (formaldehyde) can be found [[[#ese351-bib-0004|[4]]], [11], [12]], but there are no examples of direct oxidation of methane to methanol, which technically requires only a change in reactor operation. Yet, the industrial methane to methanal conversion cited was of such low capacity (<1 t/day) that it would only be referred to as large pilot-scale in current terms. To quote Arutyunov [4]:
… partial oxidation of dry natural gas, which requires more stringent conditions and gives lower yield of the target products [than heavier hydrocarbon gases], has never been mastered by the industry.
What is it then that prevents direct methane to methanol conversion to be applied?
The objective of this work was to perform an engineering evaluation of the direct methane to methanol conversion process. Of specific interest was to evaluate the potential of this process to be employed as a platform technology for small-scale gas-to-liquids (GTL) applications.
Process Chemistry
The first challenge in the direct conversion of methane is overcoming the stability of the C–H bonds in methane. Strategies to activate C–H in methane are discussed in literature, for example, [13], but activation of the C–H bond is only part of the challenge. Free radical partial oxidation is a quite successful strategy to activate C–H bonds in methane. Despite many claims in literature of the beneficial effects of employing catalysts, catalysts do not appear to offer meaningful yield advantages [[[#ese351-bib-0004|[4]]], [14]]. It appears that the role of catalysis is mainly to reduce the severity of process conditions. The partial oxidation of methane to methanol is therefore more simply conducted as a gas phase free radical process, which avoids gas cleaning to remove potential catalyst poisons. The free radical partial oxidation of methane to methanol is not deleteriously affected by the presence of hydrogen sulfide or other contaminants that may be present in natural sources of methane, such as natural gas.
The other part of the direct methane conversion challenge is to prevent further reaction of the products from partial methane oxidation. The homolytic bond dissociation energy of the C–H in methane (439 kJ · mol−1) is higher than that in methanol (402 kJ · mol−1), methanal (369 kJ · mol−1), or in methanoic acid (402 kJ · mol−1) [15]. Whatever C–H bond activation strategy, it will work better on the products of methane conversion than on methane itself. (An analogous problem presents itself for microbial conversion, where it is necessary to inhibit methanol conversion in methane to methanol processes, because methanol is more easily metabolized) [16].
If heavier hydrocarbons are present, such as ethane and propane, the reaction proceeds more readily, since it is easier to oxidize heavier hydrocarbons.
The main carbon-containing products that are produced during the partial oxidation of methane at temperatures below 450°C are methanol (eq. (1)), methanal (eq. (2)), methanoic acid (eq. (3)), carbon monoxide (eq. (4)), and carbon dioxide (eq. (5)):
|
|
(1) |
|
|
(2) |
|
|
(3) |
|
|
(4) |
|
|
(5) |
The reaction engineering strategy that is employed to maximize the methanol selectivity during gas phase oxidation of methane is to limit the single-pass conversion. This is achieved by limiting the concentration of the oxidant. When the oxidant is the limiting reagent, successive oxidation reactions are inherently limited and the selectivity to primary oxidation products is increased. Some general observations about the operating conditions and process chemistry can be made: [[[#ese351-bib-0004|[4]]], [17], [18]]
- Typical operating temperatures for noncatalytic partial oxidation of methane to methanol are in the range 370–470°C. It is impractical to perform the reaction at temperatures below 350°C, because the induction time is long and the methane oxidation rate is low. At higher temperatures, there is an increase in oxidative coupling reactions to form heavier hydrocarbons, such as ethane and ethene. No literature to the contrary was found and there is high confidence in the operating temperature range indicated.
- Methanol and other desirable products are intermediate oxidation products. Oxidation of methanol and the other oxygenates is easier than oxidation of methane. This is an example of an “A→B→C” reaction where “B” is desired product. With increasing O2 feed concentration the selectivity to methanol decreases, irrespective of the temperature. Since the O2 concentration in the feed limits the conversion, this is a typical conversion-selectivity trade-off. In order to minimize combustion reactions (eqs. (4) and (5)), it was claimed that the oxidant concentration must not exceed 2.8% and should ideally be in the range 2.0–2.8% [17]. There is also a restriction on the methane to O2 ratio, with methanol selectivity being quickly eroded as the methane to O2 ratio decreases below 30:1.
- Operating at higher pressures is beneficial to methanol selectivity and the overall yield of oxygenates (eqs. (1)-(3)). This is understandable from Le Chateliers principle, because methanol synthesis causes molar contraction, whereas the converse is true for oxidation to CO x (eqs. (4), (5)). At pressures below 8 MPa, the oxygenate yield decreases monotonously with pressure, but at pressures above 8 MPa the reaction becomes fairly insensitive to pressure. The suggested minimum practical pressure of operation is, therefore, around 8 MPa. Less was published on pressure effects and the threshold pressure value is a best estimate. There is moderate confidence in the minimum operating pressure threshold.
- In the temperature range 370–450°C at typical operating conditions described above, the selectivity to methanol is around 40%, the selectivity to methanal increases from 5% to 13% with increasing temperature and the selectivity to carboxylic acids increased from 0.5% to 0.7% with increasing temperature. There is only moderate to low confidence in the selectivity values, which represent a best estimate based on literature.
- Cofeeding free radical initiators can initiate the oxidation reaction and eliminate the induction time when employing O2 as oxidant. This strategy was employed for formaldehyde production by direct partial oxidation of methane, where a small quantity of NO was cofed [[[#ese351-bib-0004|[4]]], [11]]. Direct methane to methanol conversion making use of N2O with a catalyst to conduct the reaction at lower temperature was reported [19], and low temperature methane to methanol synthesis could also be achieved by NO catalyst activation [20].
As an alternative to oxygen as oxidant, hydrogen peroxide (H2O2) in combination with a catalyst enables low temperature oxidation of methane to methanol [21]. Other examples can also be found in the literature. Many oxidants are capable of oxidation at the carbon atom [[[#ese351-bib-0022|[22]]], [23]]. The selection of an appropriate oxidant is an economic decision, since oxidant consumption is stoichiometric with respect to the oxidation products. Air or O2 separated from air is often the most economical choice for oxidation of a total feed stream, as is the case in methane to methanol conversion.
The reaction network is complex, which is typical of any partial oxidation reaction involving a free radical mechanism. The reactions that were listed (eqs. (1)-(5)) are not a reaction network. A far more complex interrelationship exists between the various species. Not all reactions in such a reaction network require oxygen to be present. Thermal decomposition and disproportionation reactions can proceed without oxygen. For example, the thermal decomposition of methanal to produce CO, H2 and methanol (eqs. (6), (7)) [24].
|
|
(6) |
|
|
(7) |
There is no lack of information on the chemistry, but constructing a credible reaction network and obtaining kinetic data for the individual reactions are more challenging tasks. This information was conspicuously absent from discussions on the process chemistry of direct methane to methanol conversion.
Process Description
Process flow diagrams for partial oxidation of hydrocarbons with recycle, can be found in the literature [[[#ese351-bib-0004|[4]]], [9], [11], [12]]. For the purpose of an engineering evaluation, it is necessary to develop the key elements of the process more fully, so that the utility requirements can be determined. The utility footprint of GTL processes that employ synthesis gas is substantial [25]. Although the direct methane to methanol conversion process does not involve synthesis gas generation, many of the process elements leading to a high utility footprint are still present, such as oxidant compression, gas recycling, and high temperature operation.
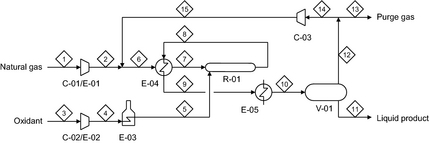
The process flow diagram of direct methane to methanol conversion is shown in Figure 2. The equipment table is given in Table 1.
|
|
|
Figure 2. Process flow diagram of direct methane to methanol conversion. |
| Number | Equipment |
|---|---|
| C-01 | Natural gas feed compressor |
| C-02 | Oxidant feed compressor |
| C-03 | Recycle gas compressor |
| E-01 | Interstage cooler for compressor C-01 |
| E-02 | Interstage cooler for compressor C-02 |
| E-03 | Oxidant heater (doubles as start-up heater) |
| E-04 | Feed-product heat exchanger |
| E-05 | Product coolers |
| R-01 | Partial oxidation reactor |
| V-01 | High-pressure phase separator vessel |
There are two raw material streams, natural gas and the oxidant. These input streams are compressed and preheated separately to avoid premature oxidation. Oxidation must be controlled to obtain good selectivity to methanol and much of the technology know-how is related to the design of the partial oxidation reactor. In the partial oxidation reactor, the methane is partially oxidized to produce methanol, with the oxidant being the limiting reagent. The product gas is cooled and the methanol is recovered by condensation. The recovered crude methanol and side-products can be separated and purified by conventional distillation. Product work-up is not unique to the direct methane to methanol synthesis process and it is not shown or considered in this evaluation. Part of the unconverted natural gas is recycled and part is purged. The purge of some unconverted natural gas is necessary to limit the build-up of inert material in the process.
The main elements of the design and design decisions associated with each will be discussed.
Natural gas feed
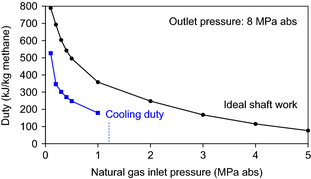
The hydrocarbon feed to the process is natural gas and not pure methane. In most instances, the natural gas will be available at an elevated pressure. The gas pressure will depend on the source of the natural gas. If it is associated gas, which is coproduced with crude oil, the gas may only be available at low pressure, for example, 0.2 MPa, while connected natural gas in a distribution network will typically be available at higher pressure, for example, 8 MPa [26]. The extent of compression and intercooling required for the process depends on the feed conditions and the calculated relationship is shown (Fig. 3). The ideal shaft work is shown, from which the actual shaft work and utility requirements can be calculated. The cooling duty was calculated based on the intercooling requirements for a realistic compressor design. Cooling duty becomes zero at an inlet pressure of ~1.2 MPa, when no intercooling is required for the compressor design.
|
|
|
Figure 3. Ideal shaft work and cooling duty for natural gas compression to 8 MPa. |
The composition of natural gas is origin dependent and the main associated gases are other hydrocarbons, carbon dioxide (CO2), nitrogen (N2), and hydrogen sulfide (H2S) [27]. Of these, nitrogen is the most difficult to separate from the unconverted natural gas, because it requires cryogenic distillation, or some form of pressure swing adsorption. Thus, of all the natural gas components nitrogen will have the most impact on the purge rate. For the purpose of this evaluation, a generic natural gas composition was employed (Table 2). This gas composition does not include H2S or Hg, which are likely to be present in some sources of natural gas [27].
| Compound | Natural gas |
|---|---|
| |
| Composition (mol fraction) | |
| Methane (CH4) | 0.950 |
| Ethane (C2H6) | 0.005 |
| Nitrogen (N2) | 0.030 |
| Carbon dioxide (CO2) | 0.015 |
| Average molecular mass (g · mol−1) | 16.89 |
| Normal gas density (kg·m−3)a | 0.754 |
| Lower heating value (MJ·m−3) | 34.3 |
| Higher heating value (MJ·m−3) | 38.0 |
Oxidant feed
It is in principle possible to use air, oxygen-enriched air, or oxygen as oxidant feed for the direct methane to methanol conversion process. The cost and the complexity of the process increase in the same order. In practice, purified oxygen is the only viable oxidant feed for the process:
- The oxidant feed is available at low pressure and the oxidant feed must also be compressed to the process pressure, around 8 MPa. The ideal shaft work for the compression of pure O2 is 400 kJ·kg−1 O2 and a realistic compressor design requires a cooling duty of 300 kJ·kg−1 O2 for interstage compressor cooling. For air, these duties change to 1.9 and 1.4 MJ·kg−1 O2, respectively.
- Any inert material introduced as part of the oxidant feed and that is difficult to separate from methane, affects the purge rate of the process. Purified O2 from cryogenic separation typically has a purity of around 99.5% O2, the remainder being argon [28]. The composition of standard dry air is 21% O2, 78% N2, and 1% Ar. Both nitrogen and argon are difficult to separate from methane. When air is used as oxidant, it increases the purge rate.
The implication of the preceding discussion is that the direct methane to methanol process requires purified O2 as oxidant feed. For smaller installations that require less than 5000 m3·h−1 normal (2 kg·sec−1) of O2, vacuum pressure swing adsorption (VPSA) can be considered, but the purity of the oxygen is only in the range 90–93% O2 [28]. For larger capacities or to reduce the impact of the inert gas in the oxidant feed, cryogenic air separation is required. This is a utility unit and it is not reflected in either Figures 1 or 2, but it clearly will have an impact on the size and cost of the process.
Liquid product
The oxygenate products, methanol, methanal, and methanoic acid (eqs. (1)-(3)), are recovered as a crude liquid aqueous product mixture by condensation under pressure. In addition to these products, some of the CO2 and H2S are removed by dissolution under pressure in the liquid phase [[[#ese351-bib-0029|[29]]], [30]]. The temperature at which phase separation is performed affects the relative amounts of products and soluble gases that are removed in the liquid phase. A small amount of the inert gases are also removed, but it is insufficient to affect the purge rate. For example, the solubility of N2 in water is about 0.1 mol % at 7 MPa [31].
Purge gas product
It was pointed out that the inert gas content of the natural gas feed and the oxidant feed both affect the purge rate. This is a generic chemical engineering problem that is encountered whenever a gas recycle is employed. The problem stems from the difficulty in separating the reactive material from the inert material. In direct methane to methanol conversion, the process chemistry dictates that a low single-pass conversion of methane is required, which necessitates the recycling of methane. Nitrogen and argon that enter the process through the natural gas feed and the oxidant feed are difficult to separate from methane. The purge is needed to ensure material balance. The same amount of N2 and Ar that enter the process must leave the process.
In practice, there are three design decisions that affect the purge rate. First, the feed composition can be selected or manipulated to ensure that the least amount of inert material enters the process. Second, a separation technology can be installed to selectively remove inert material from the recycle gas. This is unlikely to be a practical option for this process. The separation of methane from N2 and Ar requires cryogenic distillation with prior CO2 removal to avoid solid CO2 plugging of the cryogenic section [28]. Third, the concentration of the inert material in the recycle gas can be manipulated.
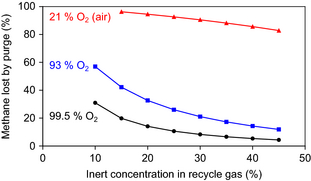
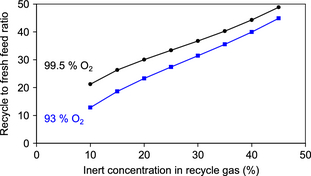
The impact of the inert composition of the oxidant feed and the inert concentration in the recycle gas on the purge rate of methane was calculated and it is illustrated by Figure 4. In the calculations, the natural gas feed composition in Table 2 and 30:1 methane to O2 feed ratio were employed. For 99.5% O2 from cryogenic separation the least amount of methane is lost through the purge. For air, which has only 21% O2, the methane loss through the purge is substantial, even at a high inert concentration in the recycle gas.
|
|
|
Figure 4. Impact of O2 purity in oxidant feed and inert concentration in the recycle gas on the methane loss through the purge gas. |
The calculated methane loss through the purge gas (Fig. 4) is also affected by the inert content in the natural gas feed. The effect of the inert content in the natural gas feed decreases in significance as its contribution to the overall inert content entering the process decreases. For example, if the N2 content in the natural gas is 1% instead of 3% (Table 2), the methane loss with a 99.5% O2 feed is 60% smaller than that indicated in Figure 4, but with a 93% O2 feed the methane loss is decreased by only 20%. The methane loss is determined by the total amount of inert material that enters the process.
The methane in the purge gas can be employed as fuel gas in the process. Since the volumetric heating value of the purge gas decreases with increasing inert concentration, there is a trade-off between the fuel gas needs, fuel gas quality and the design of the gas recycle.
Gas recycle
The size of the gas recycle determines the sizing of equipment in the gas loop. With reference to Figure 2, the equipment in the gas loop is the partial oxidation reactor (R-01), feed-product heat exchanger (E-04), product coolers (E-05), phase separator vessel (V-01), and recycle gas compressor (C-03).
The gas recycle is particularly large in comparison to the fresh feed streams due to the low single-pass conversion. For an ideal reactor and separator with no inert materials in the feed, the ratio of the recycle flow rate to the fresh feed flow rate can be calculated in terms of the single-pass conversion, x (eq. (8)):
|
|
(8) |
In the direct methane to methanol process, the single-pass conversion is limited to around 3% by limiting the oxidant in the feed to the partial oxidation reactor. A recycle to fresh feed ratio of ~30:1 is anticipated from equation (8), the exact ratio depending on the efficiency of separation and the impact of the purge rate. The recycle to fresh feed ratio increases as the design concentration of inert material in the gas recycle is increased. The recycle to fresh feed ratio also increases as the inert content in the feed materials decrease, because the system becomes more efficient and less methane is lost through the purge (i.e., not recycled). The calculated impact of O2 purity on the inert concentration in the recycle is shown in Figure 5. A lower recycle ratio does not necessarily imply that in absolute terms the flow rate through the partial oxidation reactor is less, because purge losses are compensated for by an increased fresh feed flow rate.
|
|
|
Figure 5. Impact of O2 purity in oxidant feed and inert concentration in the recycle gas on the recycle to fresh feed ratio of the process. |
Partial oxidation reactor
Product selectivity depends on temperature, pressure, O2 concentration, and residence time. The proposals for reactor designs and improvements in reactor designs are based partly on an appreciation of the reaction variables that need to be controlled and on empirical observations. It was pointed out that a well-founded reaction network with kinetic data is conspicuously absent from the literature dealing with direct methane to methanol conversion. To add to this uncertainty, reaction rate and selectivity are affected by the construction material of the reactor [[[#ese351-bib-0004|[4]]], [18]].
Some general observations about reactor design can be made that agree with experimental observations and reactor designs suggested for this process.
- Temperature will affect the reaction rate of oxidation, but it will also affect the contribution of thermal decomposition. Since partial oxidation reactions are exothermic (Table 3), it is preferable to limit the temperature, in particular to avoid loss of methanal (eqs. (6), (7)) even though some methanal is converted to methanol by thermal decomposition. Heat management is important.
- Control of the residence time is not independent of temperature. There are three aspects to consider. First, thermal decomposition reactions can take place independently of the other species present and there will be a threshold temperature below which these reactions become negligible. Control of the residence time, independent of oxygen consumption, is therefore important when the temperature is high enough for thermal decomposition reactions to take place. Second, yield of an intermediate product in a simple reaction is determined by the rate constant of the formation of the intermediate product (k1) in relation to the rate constant for the consumption of the intermediate product (k2). Since these reactions typically have different activation energies, the ratio of k1/k2 is dependent on the temperature. In a complex reaction network, the time–temperature relationship becomes important, because it is the global optimum that must be achieved, not the optimum for a single product. Third, there is an induction period in free radical oxidation that can be shortened or eliminated by the use of an initiator. During the induction period, oxidation is negligible. The length of the induction period is dependent on the temperature and it is actually the time–temperature profile once O2 comes into contact with the methane that determines the length of the induction period. There is of course also an implicit risk for the reactor design if the absence of initiators in the reactor feed cannot be guaranteed.
- Oxygen availability determines product selectivity. The methane to O2 ratio threshold assumes a well-mixed system. What this implies is that after mixing the methane and O2, the mixture must be homogenized before the end of the induction period.
| Product | Equation | ΔHr (kJ · mol−1) |
|---|---|---|
| Methanol | 1 | −127 |
| Methanal (formaldehyde) | 2 | −276 |
| Methanoic acid (formic acid) | 3 | −546 |
| Carbon monoxide | 4 | −519 |
| Carbon dioxide | 5 | −802 |
The detailed reactor design is very important. Although the reactor is not very complex, the design of the reactor is very complex. Scale-up without a fundamental description of the reaction network and the flow dynamics is likely to involve trail-and-error. In this respect, history serves as lesson. The oxygenate yield that was predicted Gutehoffnungschütte-process for direct methane to methanal conversion was 35%, but on scale-up the actual yield was only around 10% [4].
Process Evaluation
Material balance
A material balance for the process (Table 4) was calculated based on the natural gas composition in Table 2 and a natural gas feed rate of 1 kg · sec−1. The feed basis is equivalent to a volumetric natural gas flow rate at standard conditions of 115,000 m3 per day (4 million scf per day). With reference to Figure 2, other important assumptions and design decisions that were made in the preparation of the material balance are:
| Compounds | Flow rate (kg · sec−1)a | ||||||
|---|---|---|---|---|---|---|---|
| Gas feed | Oxidant feed | Reactor feed | Reactor product | Liquid product | Purge gas | Recycle | |
| #1 | #3 | #7 | #8 | #11 | #13 | #15 | |
| |||||||
| CH4 | 0.902 | – | 25.485 | 24.892 | – | 0.309 | 24.583 |
| C2H6 | 0.009 | – | 0.009 | 0.000 | – | 0.000 | 0.000 |
| O2 | – | 1.514 | 1.551 | 0.038 | – | 0.000 | 0.037 |
| N2 | 0.050 | – | 3.999 | 3.999 | – | 0.050 | 3.949 |
| Ar | – | 0.009 | 0.764 | 0.764 | – | 0.009 | 0.755 |
| CO | – | – | 0.409 | 0.414 | – | 0.005 | 0.409 |
| CO2 | 0.039 | – | 3.035 | 3.792 | 0.758 | 0.038 | 2.996 |
| H2O | – | – | 0.000 | 0.716 | 0.716 | 0.000 | 0.000 |
| Alcohols | – | – | 0.000 | 0.480 | 0.479 | 0.000 | 0.000 |
| Aldehydes | – | – | 0.000 | 0.146 | 0.146 | 0.000 | 0.000 |
| Carboxylic acids | – | – | 0.000 | 0.012 | 0.012 | 0.000 | 0.000 |
| Σ (kg · sec−1) | 1.000 | 1.523 | 35.252 | 35.252 | 2.111 | 0.412 | 32.729 |
| Molar flow (mol · sec−1) | 59.2 | 47.5 | 1882.8 | 1882.8 | 77.0 | 22.4 | 1776.0 |
- The natural gas feed (stream 1) is available at 25°C and 2.0 MPa absolute. In practice, this value will be dependent on the nature of the natural gas resource. For example, natural gas from deep reservoirs will have a much higher pressure, whereas associated natural gas available after crude oil depressurization may be available at lower pressure [26].
- The recycle (stream 15) to fresh feed (stream 1) ratio was fixed at 30:1 on a volumetric basis. It is not claimed that this is the optimum, but it provides a credible trade-off between recycling, inert content in the recycle and purging rate. In principle this number can be increased, but not by much, otherwise it invalidates the constraints on reactor operation for the product selectivity values employed.
- The oxidant feed (stream 3) is 99.5% O2 from a cryogenic air separation unit. The only inert introduced in this way is 0.5% Ar. The oxidant is available at 25°C and 0.1 MPa absolute.
- The oxidant feed rate was fixed at 2.5% O2 concentration on a volumetric basis in the combined reactor feed. This value resulted in a methane to O2 ratio just over 30:1.
- The reactor inlet conditions are 8 MPa and 370°C.
- The overall pressure drop between the feed supply and product recovery is 500 kPa.
- Recoveries for the oxygenate products in the liquid phase is 99.9%. This is based on high-pressure recovery at 25°C by phase separation (V-01).
- The CO2 removal due to dissolution in the aqueous product is 20% based on partial pressure of CO2 of the system [30]. Although some of the other gases also dissolve to some extent in the liquid product, these contributions were neglected.
- Conversion of O2 in the reactor (R-01) is complete.
- Conversion of CO present in the recycle gas to the reactor is complete. The ratio of CO:CO2 produced by partial oxidation of methane was assumed to be 85:15 based on typical values from literature [[[#ese351-bib-0004|[4]]], [18]].
- The product selectivities are based on what has been claimed in literature [[[#ese351-bib-0004|[4]]], [17]]. Both material and atom balance closure was ensured. Water production was calculated under the assumption that no H2 will be produced. The molar ratio of water to methanol reported was 2.4 [4], and was reasonably close to the value of 2.7 obtained from material and atom balance closure.
- Ethane in the natural gas feed is converted into ethanol, ethanal (acetaldehyde), and ethanoic acid (acetic acid). Conversion of ethane is assumed to be complete, because it is easier to convert heavier hydrocarbons than methane.
The natural gas feed and oxidant feed flow rates are of the same order of magnitude, but even combined, these flow rates are an order of magnitude smaller than the recycle (Table 4). The low single-pass methane conversion and the need for a large methane recycle dominates the material flow in the process. The material balance supports the qualitative predictions that were made using simplified calculations (Fig. 5). Likewise, the methane loss through the purge is substantial, as qualitatively predicted using simplified calculations (Fig. 4).
Energy and utility use
The main energy and utility consumers in the direct methane to methanol process are the compressors, heat exchangers, and air separation unit. Additional energy and utilities are required during product purification and treatment of waste streams, but as indicated before, these units were excluded from the scope of the evaluation.
The energy and utility consumption for the process is given in Table 5. The values for utility use are influenced by the process design and equipment selection.
| Equipment or unit | Duty (MW)a | Power (MW)b | Utilities (kg · sec−1)c | ||
|---|---|---|---|---|---|
| Natural gas | Cooling water | Steamd | |||
| |||||
| Natural gas feed compressor (C-01) | 0.29 | 0.79 | 0.017 | – | – |
| Oxidant feed compressor (C-02) | 0.75 | 2.03 | 0.045 | – | – |
| Recycle gas compressor (C-03) | 0.35 | 0.94 | 0.021 | – | – |
| Interstage cooler for C-01 (E-01) | – | – | – | – | – |
| Interstage cooler for C-02 (E-02) | 0.46 | – | – | 5.4 | – |
| Oxidant heater (E-03) | 0.35 | 0.41 | 0.009 | – | – |
| Feed-product heat exchanger (E-04) | 31.02 | – | – | – | – |
| Product coolers (E-05) | 7.20 | – | – | 86.0 | – |
| Partial oxidation reactor (R-01) | 7.89 | – | – | – | −3.7 |
| Air separation unit | 0.88 | 2.38 | 0.052 | 4.1 | – |
| Σ | 0.144 | 95.5 | −3.7 | ||
Due to the potential application of this process for small-scale GTL, reciprocating compressors, rather than axial compressors might be a more realistic selection [32]. A compression efficiency of 0.9 and a mechanical efficiency of 0.9 were employed for shaft work calculations. The use of natural gas powered internal combustion engines is more practical for a small-scale GTL application since the infrastructure for delivering the required amount of electric power might not be available [33]. The power consumption was converted into a natural gas consumption for internal combustion engine drives, with a drive efficiency of 0.37 [34]. As an alternative, the purge gas can be employed instead of natural gas, albeit with some loss in efficiency. The purge gas is 86 vol % methane with a lower heating value (LHV) of 31.0 MJ · m−1 and higher heating value (HHV) of 34.4 MJ · m−3.
The heat exchanger network was not optimized and it is likely that a slightly lower utility use can be achieved by better heat integration. One fired heater is necessary for start-up, even though a steady-state optimization will show that it is not necessary. Product cooling (E-05) is shown as a single exchanger, but in practice it will be multiple exchangers, which was considered in the calculations. The assumption was made that all cooling duty is supplied by cooling water, although it is likely that in practice air coolers will be used for some of that cooling duty. It was further assumed that cooling water is available at 20°C and that 40°C was an acceptable cooling water return temperature. For small-scale GTL application, a closed cooling water system is likely to be more practical, albeit more expensive, since sufficient fresh water might not be available to allow evaporative cooling [33].
Although product purification was outside of the scope of the evaluation, some steam will be required for distillation. Low pressure steam, 0.5 MPa absolute, was produced where practical.
The work required by the air separation unit to produce 99.5% O2 is 0.35 kWh per normal m3 of unpressurized oxygen [28]. This is equivalent to 0.88 MW · kg−1 O2. The air separation unit also requires cooling water for the initial cooling of the outlet of the primary air compressor [28]. Using the same utility assumptions as for the rest of this evaluation, the required cooling water flow is 4.1 kg · kg−1 O2.
It is best to view the utility requirements in Table 5 as indicators of the utility footprint. In an optimized design the absolute numbers will differ, but the numbers will be of the same order of magnitude. The process requires much cooling capacity and power for gas compression. Compared to the natural gas feed, an additional 14% natural gas is required for heating and power. The cooling water flow rate is 95 kg · kg−1 natural gas, that is, almost two orders of magnitude more on a mass flow basis.
A large utility footprint is a common feature of GTL facilities and one of the challenges in the development of a credible process configuration for small-scale GTL applications [[[#ese351-bib-0025|[25]]], [35]].
Process efficiency
There are various metrics that can be used to assess the efficiency of processes. Two of the most commonly employed are carbon efficiency and thermal efficiency. The carbon efficiency is defined as the fraction of the carbon in the feed to the process that is contained in useful products. The thermal efficiency is defined as the fraction of the LHV of the feed to the process that is retained by the useful products and it is more often employed when the products are fuels. In both instances, it is important to be clear on the system boundary. These values can be calculated for the process streams only, or they can be calculated for the overall process. When the process is not energy intensive there will be little difference in the calculated efficiencies, but not for energy intensive processes. For example, the carbon efficiency of indirect methanol synthesis based on the process streams only is better than 95% [3], but for the overall process it is 65–68% [9]. Somewhat lower efficiency is likely if the indirect methanol synthesis process is scaled down.
The overall process efficiency was assessed by comparing the thermal efficiency and the carbon efficiency of the direct methane to methanol conversion with indirect methanol synthesis (Table 6) [9]. In the calculation of the overall efficiency of the direct methane to methanol process it was assumed that the purge gas can be used as fuel gas to supply all of the energy of compression and heating requirements (Table 5), so that no additional natural gas is consumed for these purposes.
| Description | Overall process efficiency (%) | |
|---|---|---|
| Carbon | Thermal | |
| Direct methane to methanol | 35 | 28 |
| Indirect methanol synthesis | 65–68 | 51–54 |
Strictly speaking the comparison in Table 6 is not on the same basis, because the efficiency values for indirect methanol synthesis include carbon and energy losses from product purification, and it is at larger scale. However, the direct methane to methanol process has excess heating value available as steam and from the use of the purge gas as fuel gas. However, the difference between the two processes is so large that the difference in scale and battery limit does not change the conclusion.
It is clear that the overall process efficiency of indirect methanol synthesis is much higher than that of direct methane to methanol conversion. The main reason for this is reaction selectivity. During direct methane to methanol synthesis, the production of CO and CO2 (eqs. (4), (5)) is undesirable and these reactions erode carbon efficiency. Conversely, during indirect methanol synthesis, CO and CO2 are potential product-forming reactants. The single-pass conversion during indirect methanol synthesis is equilibrium limited and not limited by side-reactions. In fact, the selectivity to methanol even at near equilibrium conversion is of the order of 99% [2]. Thus, almost no carbon that enters the gas loop during indirect methanol synthesis is lost due to irreversible side-reactions, whereas the irreversible carbon loss due to side-reactions during direct methane to methanol synthesis is 40–50% (46% based on the assumptions used in this work). Based on the calculated efficiencies (Table 6), direct partial oxidation of methane to methanol must achieve a combined methanol and methanal selectivity of the order 90% to become competitive with indirect methane to methanol synthesis.
Discussion
The objective of the present investigation was to perform an engineering evaluation of the process and evaluate the potential of this process for small-scale GTL applications.
There is reasonable agreement between the heat transfer and compression duties reported by Kuo [9], and that calculated during the present investigation. These duties cannot be avoided even though more optimized heat integration would change the numeric values somewhat. However, the overall outcome of the evaluation was more pessimistic than the evaluation by Kuo. This is primarily due to a difference in process chemistry, with Kuos analysis being based on the optimistic methanol selectivity of 90% and 15% single-pass methane conversion. These optimistic values were based on the work by Gesser, Hunter, and coworkers [36]. The bulk of the literature suggests that such methanol selectivities are not obtained [4]. There are also fundamental reasons why such optimistic data are questionable, notably the implicit adiabatic temperature increase and the relative ease of further oxidation of methanol compared to oxidation of methane.
One of the major disappointments from this study was that a credible engineering design for the direct methane to methanol process required high purity O2. The anticipated cost and complexity advantage over indirect methane to methanol synthesis could, therefore, not be realized, because an air separation unit was required. Hence, for small-scale GTL applications, direct methane to methanol had no specific advantage over indirect methane to methanol. When this is combined with the low methanol selectivity and low single-pass methane conversion, there is little technical incentive to justify further interest in direct oxidation of methane as a potential process for methanol synthesis. Furthermore, based on the fundamentals of gas recycling and the fundamentals of free radical oxidation, it is doubtful that direct methane to methanol conversion can ever be competitive with indirect methane to methanol conversion.
Conclusions
The objective of the present investigation was to perform an engineering evaluation of the direct methane to methanol conversion process and evaluate the potential of this process for small-scale GTL applications. The most noteworthy observations from the engineering evaluation were:
- An air separation unit was required to provide high purity O2 as oxidant. The process cannot be operated with air as the oxidant feed. The need for an air separation unit diluted the benefit of avoiding synthesis gas generation as a process step (Fig. 1). If one considers synthesis gas generation by autothermal reforming and its associated air separation unit, only part of the complexity and cost is avoided by direct methane to methanol conversion.
- Natural gas is not pure methane and oxygen from air separation is not pure O2. The introduction of some inert gases is unavoidable and this affects the design. The recycle ratio, capacity of the gas loop, and purge gas loss all depend on the amount of inert gases introduced in the feed.
- The utility footprint of the process is significant, with compressor and cooling duties dominating utility requirements. This is typical of GTL processes. The large utility footprint of GTL processes is one of the key challenges for small-scale GTL applications and in this respect direct methane to methanol conversion offers little benefit.
- Overall the carbon efficiency is 35% and the thermal efficiency is 28%, which is about half of that of indirect methanol synthesis (65–68% and 51–54%, respectively).
- Low oxygenate selectivity during direct partial oxidation of methane is the largest contributor to the loss of process efficiency compared to indirect synthesis of methanol. The process chemistry is burdened by some fundamental constraints. First, the reactivity of methanol and methanal (desirable products) to further oxidation is higher than that of methane. Second, there are thermal reactions that can proceed in the absence of the oxidant to decrease oxygenate selectivity, so that limiting oxidant availability on its own is insufficient to achieve better selectivity. Third, CO and CO2 are fatal products and these products represent an irreversible carbon loss.
Conflict of Interest
None declared.
References
- Tijm, P. J. A., F. J. Waller, and D. M. Brown. 2001. Methanol technology developments for the new millennium. Appl. Catal. A221:275–282.
- Lee, S.1990. Methanol synthesis technology. CRC Press, Boca Raton.
- Rostrup-Nielsen, J., and L. J. Christiansen. 2011. Concepts in syngas manufacture. Imperial College Press, London.
- Arutyunov, V.2014. Direct methane to methanol. Elsevier, Amsterdam.
- De Klerk, A., and V. Prasad. 2012. Methane for transportation fuel and chemical production. Pp. 327–384inT. M. Letcher and J. L. Scott, eds. Materials for a sustainable future. Royal Society of Chemistry, Cambridge, UK.
- Alvarez-Galvan, M. C., N. Mota, M. Ojeda, S. Rojas, R. M. Navarro, and J. L. G. Fierro. 2011. Direct methane conversion routes to chemicals and fuels. Catal. Today171:15–23.
- Holmen, A.2009. Direct conversion of methane to fuels and chemicals. Catal. Today142:2–8.
- Lunsford, J. H.2000. Catalytic conversion of methane to more useful chemicals and fuels: a challenge for the 21st century. Catal. Today63:165–174.
- Kuo, J. C. W.1992. Engineering evaluation of direct methane conversion processes. Pp. 483–526inE. E. Wolf, ed. Methane conversion by oxidative processes. Van Nostrand Reinhold, New York.
- Kuo, J. C. W., C. T. Kresge, and R. E. Palermo. 1989. Evaluation of direct methane conversion to higher hydrocarbons and oxygenates. Catal. Today4:463–470.
- Asinger, F.1968. Paraffins chemistry and technology. Pergamon Press, Oxford.
- Sittig, M.1962. Combining oxygen and hydrocarbons for profit. Gulf Publishing Co., Houston.
- Hubert, A. J.1983. Methane. Pp. 245–261inW. Keim, ed. Catalysis in C1 chemistry. D. Riedel Publishing Company, Dordrecht.
- Gesser, H. D., and N. R. Hunter. 1992. The direct conversion of methane to methanol (DMTM). Pp. 403–425inE. E. Wolf, ed. Methane conversion by oxidative processes. Van Nostrand Reinhold, New York.
- Blanksby, S. J., and G. B. Ellison. 2003. Bond dissociation energies of organic molecules. Acc. Chem. Res.36:255–263.
- Han, J.-S., C.-M. Ahn, B. Mahanty, and C.-G. Kim. 2013. Partial oxidative conversion of methane to methanol through selective inhibition of methanol dehydrogenase in methanotrophic consortium from landfill cover soil. Appl. Biochem. Biotechnol.171:1487–1499.
- Pawlak, N. A., V. I. Vedeneev, and R. W. Carr. Method and apparatus for producing methanol. Patent US 8293186, 2012.
- Shtern, V. Y.1964. The gas phase oxidation of hydrocarbons. Pergamon Press, New York.
- Starokon, E. V., M. V. Parfenov, S. S. Arzumanov, L. V. Pirutko, A. G. Stepanov, and G. I. Panov. 2013. Oxidation of methane to methanol on the surface of FeZSM-5 zeolite. J. Catal.300:47–54.
- Sheppard, T., C. D. Hamill, A. Goguet, D. W. Rooney, and J. M. Thompson. 2014. A low temperature, isothermal gas-phase system for conversion of methane to methanol over Cu-ZSM-5. Chem. Comm.50:11053–11055.
- Hammond, C., N. Dimitratos, R. L. Jenkins, J. A. Lopez-Sanchez, S. A. Kondrat, A. Hasbi, et al. 2013. Elucidation and evolution of the active component within Cu/Fe/ZSM-5 for catalytic methane oxidation: from synthesis to catalysis. ACS Catal.3:689–699.
- Chinn, L. J.1971. Selection of oxidants in synthesis. Oxidation at the carbon atom; Marcel Dekker, New York.
- Hudlický, M. 1990. Oxidations in organic chemistry (ACS Monograph Ser. 186). American Chemical Society, Washington DC.
- Steacie, E. W. R. 1954. Atomic and free radical reactions. 2 ed. (ACS Monograph Ser. 125), Reinhold Publishing Corporation, New York, NY.
- Zennaro, R.2013. Fischer-Tropsch process economics. Pp. 149–169inP. M. Maitlis and A. De Klerk, eds. Greener Fischer-Tropsch processes for fuels and feedstocks. Wiley-VCH, Weinheim.
- Szilas, A. P.1986. Production and transport of oil and gas. 2 ed. Elsevier, Amsterdam.
- Peebles, M. W. H.1992. Natural gas fundamentals. Shell, London.
- Häring, H. -W. ed. 2008. Industrial gases processing. Wiley-VCH, Weinheim.
- Carroll, J. J., and A. E. Mather. 1989. The solubility of hydrogen sulphide in water from 0 to 90°C and pressures to 1 MPa. Geochim. Cosmochim. Acta53:1163–1170.
- Mason, D. M., and R. Kao. 1980. Correlation of vapor-liquid equilibria of aqueous condensates from coal processing. ACS Symp. Ser.133:107–138.
- Chapoy, A., A. H. Mohammadi, B. Tohidi, and D. Richon. 2004. Gas solubility measurement and modeling for the nitrogen + water system from 274.18 K to 363.02 K. J. Chem. Eng. Data49:1110–1115.
- Bloch, H. P.2006. A practical guide to compressor technology. 2 ed. Wiley-Interscience, Hoboken, NJ.
- De Klerk, A. 2014a. Consider technology implications for small-scale Fischer-Tropsch GTL. Gas ProcessJuly/August:41–48.
- Ulrich, G. D., and P. T. Vasudevan. 2004. Chemical engineering process design and economics. A practical guide. 2 ed. Process Publishing, Durham, NH.
- De Klerk, A.2014b. Small-scale gas-to-liquids using Fischer-Tropsch synthesis: opportunity or myth?. Prepr. Pap. -Am. Chem. Soc. Div. Energy Fuels59:823–824.
- Gesser, H. D., N. R. Hunter, and C. B. Prakash. 1985. The direct conversion of methane to methanol by controlled oxidation. Chem. Rev.85:235–244.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?