(Created page with " ==Abstract== Production of biogas utilizing agricultural wastes is one of the most demanding technologies for generating energy in sustainable manner considering environmen...") |
m (Scipediacontent moved page Draft Content 521638083 to Corro et al 2014a) |
(No difference)
| |
Latest revision as of 14:09, 1 June 2017
Abstract
Production of biogas utilizing agricultural wastes is one of the most demanding technologies for generating energy in sustainable manner considering environmental concerns. However, though the agricultural wastes are available in abundance, the technologies used for the production of biogas such as biological and thermochemical processes are not very efficient. In the present article, we describe a process for the production of biogas from coffee pulp utilizing Cu/TiO2 as an efficient photocatalyst in its solar photocatalytic pretreatment, producing biogas of high caloric value at enhanced rate. The pretreatment process enhances the coffee pulp–cattle manure codigestion, producing increased amounts of methane, propane, and other combustible components of biogas. The photocatalytic pretreatment was performed using 10%Cu/TiO2 as photocatalyst, bubbling air as oxidizing agent, and solar radiation as light source. The process enhances the degradation rate of lignocellulosic components of coffee pulp, consequently increasing the biogas production through anaerobic codigestion. The mechanism of photocatalytic degradation of lignocelluloses has been discussed. The results presented in this work indicate the photocatalytic pretreatment is a useful process to increase biogas generation from lignocelluloses-rich natural wastes.
Introduction
Methane production from a variety of biological wastes through anaerobic digestion technology is growing worldwide and is considered ideal in many ways due to its economic and environmental benefits [1-10]. Methane fermentation is the most efficient technology for energy generation from biomass in terms of energy output/input ratio (28.8 MJ/MJ) among all the technologies used for energy production through biological and thermochemical routes [11].
Coffee is the second largest traded commodity in the world which generates large amounts of by-products and residues during processing. Industrial processing of coffee cherries is performed to separate coffee beans by removing shell and mucilaginous part. In wet industrial processes, a large amount (about 29% dry-weight of the whole coffee berry) of coffee pulp is produced as the first by-product. The organic components present in coffee pulp include cellulose (63%), lignin (17%), proteins (11.5%), hemicelluloses (2.3%), tannins (1.80–8.56%), pectic substances (6.5%), reducing sugars (12.4%), nonreducing sugars (2.0%), caffeine (1.3%), chlorogenic acid (2.6%), and caffeic acid (1.6%) [12-14]. Coffee wastes and by-products produced during coffee berry processing constitute a source of severe contamination and pose serious environmental problems in coffee-producing countries. Therefore, disposal of coffee pulp is becoming an emerging environmental problem worldwide due to its putrefaction. Due to anaerobic conditions of open pulp-storage or composting areas, an uncontrolled emission of methane (CH4) and nitrous oxide (N2O) from these places cannot be excluded [15-17]. Hence, the utilization and management of coffee wastes in large scale still remains a challenge worldwide not only due to the generation of earlier gases but also for their high contents of caffeine, free phenols, and tannins, which are known toxic agents for many biological processes [18].
Use of anaerobic digestion process for the treatment of organic fraction of municipal solid wastes has been popular worldwide during the past decades. Indeed, the biological degradation process of organic materials under anoxic conditions lead to the production of methane, which can be used as an efficient renewable energy source in comparison with aerobic stabilization processes that require higher energy consumption [19].
Codigestion can be defined as the simultaneous digestion of two or more biomass wastes which contribute to different organic matter. The benefits of codigestion include dilution of potential toxic compounds, improved balance of nutrients, synergistic effect of microorganisms, higher loading of biodegradable organic matter, and higher biogas yield [20].
Generation of methane from solid organic waste through anaerobic digestion is significantly affected by the mass transfer in each of the involved biological steps, as well as by the availability of biodegradable organic matter [21]. The most important step in this regard is the hydrolysis of complex organic molecules to soluble compounds. Several physical [22, 23], chemical [24, 25], and biological processes [26, 27], or their combinations have been utilized to improve the efficiency of anaerobic treatments [28, 29].
Lignocellulosic biomass is the major structural component of wood and plant fibers, which is also the most abundant polymer synthesized by nature. However, its use as a feedstock for fuels and chemicals has been limited due to its highly crystalline structure, inaccessible morphology, and limited solubility. Moreover, it is difficult to degrade biologically. Pretreatment of the agricultural residues by mechanical size reduction, heat treatment, and/or chemical treatment usually improves its digestibility. Explored chemical pretreatment methods include bicarbonate treatment [30], alkaline peroxide treatment [31], ammonia treatment [32]. Electron-beam radiation has also been used as pretreatment of biomass to improve its digestibility [33-36]. However, these pretreatment methods are complicate, need special equipment, and hence not cost-effective.
On the other hand, application of the photocatalytic method for the destructive removal of natural organic substances has been extensively studied [37, 38]. As example, several researchers have utilized TiO2 under UV illumination to decompose refractory lignin [38, 39]. Tanaka et al. have applied TiO2 and UV irradiation to degrade wastewater lignin [40]. The time for complete delignification could be shortened by increasing the amount of TiO2. On the other hand, incorporation of noble metal into TiO2 has also seen to enhance its photocatalytic efficiency for the degradation of refractory compounds [41-45].
In general, photocatalytic oxidation can be considered as an example of innovative technologies collectively known as advanced oxidation processes (AOP) that rely on the generation of highly reactive radicals. Those reactive species are subsequently used to degrade organic pollutants. The principles of photocatalytic oxidation and its environmental applications have been reviewed extensively [36-43, 46]. Basically, the heterogeneous photocatalytic processes depend on the utilization of UV or near-UV radiation to photo-excite the semiconductor catalysts in presence of oxygen. Under these circumstances, oxidizing species such as surface-bound hydroxyl radical (·OH), superoxide (·O2−), hydroperoxy radical (HO2·), and free holes are generated. The active species could initiate a series of redox reactions to degrade adsorbed molecules [47]. Of the several semiconducting oxides utilized as photocatalysts, TiO2 in anatase phase has seen to be the most efficient due to its high stability, high photocatalytic efficiency, low toxicity, and low cost. High optical absorbance in the near-UV region remains the major advantage of TiO2 for this purpose. However, due to the technical problem arising from the dissolution/dispersion of TiO2, it is difficult to separate from the solvent or reaction mixture. While this is a practical problem for the reuse of the catalyst, the presence of fine TiO2 particles in the supernatant complicates the estimation of catalytic products.
In this investigation, we have prepared a Cu/TiO2 catalyst, which is of semiconducting nature, having strong absorbance in between 235 and 400 nm, and an intense absorption band in the visible region (400–800 nm), suggesting its utilization for photocatalytic degradation of the complex organic substances present in waste coffee pulp. The photocatalytic degradation of lignin may generate simple organic molecules, which may be suitable for further anaerobic digestion. The photocatalytic activity of the calcined Cu/TiO2 catalyst under direct solar radiation for enhanced solubility of refractory compounds of lignocellulosic biomass present in coffee pulp has been studied. The use of solar energy as UV–vis irradiation source brought down the biogas production cost in comparison with the other biomass pretreatment methods developed to date [30-36]. To the best of our knowledge, there is no report in the literature so far on the photocatalytic degradation of lignocellulosic biomass contained in coffee pulp.
Materials and Methods
Collection and preparation of substrates
Our experiments were performed with coffee pulp collected during the coffee bean processing in a semitropical region of Puebla, Mexico. The cattle manure was collected from a dairy farm.
Catalyst preparation
The Cu/TiO2 catalyst was prepared by the impregnation method using titanium dioxide (Baker 99.99%) and appropriate amounts of aqueous solution of Cu(NO3)2 (Aldrich 99.99%) to obtain a 10 wt% Cu on the TiO2. The suspension was stirred at room temperature for 4 h. After drying at 120°C overnight, the sample was calcined in air at 800°C for 4 h, after which it is called as 10%Cu/TiO2 catalyst. After calcination, the catalyst presented a very high indentation hardness and resistance to pulverization. A reference TiO2 support was prepared in the same way using only distilled water (without Cu(NO3)2).
Catalyst characterization
Adsorption/desorption isotherm of the samples were measured using a Quantachrome Nova-1000 sorptometer (Quantachrome Instruments, Florida, USA). Specific surface area (Sg) of the samples was estimated from their N2 physisorption at 77 K, using BET analysis methods.
The diffuse reflectance spectra (DRS) of the composite catalyst and the reference sample were obtained for their dry-pressed disks (~15 mm diameter) using a Varian Cary 500 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) with DRA-CA-30I diffuse reflectance accessory using BaSO4 as standard reflectance sample. The crystallinity and structural phase of the samples were verified through powder X-ray diffraction (XRD) technique using the Cu Kα source (λ = 1.5406 Å) of a Bruker D8 Discover diffractometer (Bruker Corporation, Bruker, Mexico).
Photocatalytic pretreatment of coffee pulp
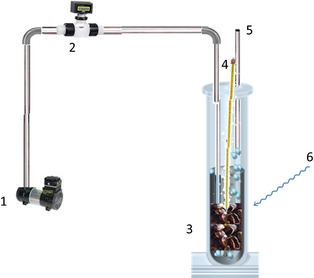
The photocatalytic pretreatment of coffee pulp was carried out in a quartz photoreactor, newly designed and built in our laboratory. The detailed schematic drawing of the experimental setup is shown in Figure 1.
|
|
|
Figure 1. Schematic layout of the photoreactor used for the photocatalytic pretreatment of coffee pulp. 1: air compressor; 2: mass flow controller; 3: coffee pulp, water, and catalyst mixture; 4: thermometer; 5: air outlet; 6: solar radiation exposure. |
The sunlight was utilized as UV radiation source to expose the catalytic reaction mixture. Ambient air from a compressor was fed into the reactor at the rate of 0.5 L min−1.
The quartz reactor (called reactor A) of 10 cm inner diameter was filled with a mixture of 10%Cu/TiO2 (100 g), coffee pulp (100 g) and water (500 mL). Small transparent fabric sachets were used as catalyst containers, which permitted a total and immediate separation of catalyst from the reaction mixture after the photocatalytic pretreatment process.
A second reactor (called reactor B) containing a similar reaction mixture without the catalyst was also studied under the same solar irradiation and air-flow conditions. The temperature of the reactor was maintained at 30°C. The reactors were exposed to solar radiation from 9:00 h to 16:00 h during 30 days (April). On average, the intensity of the exposed solar radiation was about 1000 W m−2. After these 30 days of exposure, the catalyst was separated from the reaction mixture, which was transferred to the digester used for anaerobic codigestion tests.
The catalyst was easily separated from the mixture due to its high crystallinity and indentation hardness. However, the possibility of catalyst leaking or diluting in the reaction mixture cannot be discarded. In order to probe that no residual photocatalyst remained in the reaction mixture, after the photocatalytic pretreatment, we analyzed this mixture by atomic absorption spectrometry, using an AA-7000 spectrophotometer provided by Shimadzu (Shimadzu Corporations, Tokyo, Japan). Results revealed a Cu concentration lower than 2 ppm in the sample. This low-level indicates a very low leaching of 10%Cu/TiO2 catalyst during photocatalytic pretreatment.
Anaerobic codigestion tests
Preliminary tests for the optimization of catalyst/cattle manure ratio
To optimize the photocatalytically pretreated coffee pulp/cattle manure ratio in the digesters, we performed some simple tests in small stainless steel vessels with different weight ratios of photocatalytically pretreated coffee pulp and cattle manure. The volume of the test vessels was 0.1 L, with a working volume of about 0.05 L. About 1 g of the photocatalytically pretreated coffee pulp and cattle manure mixture with different (0:1, 0.5:1, 1:1, 2:1, and 1:0) weight ratios were incorporated into the test vessels along with 0.025 L of water. The test digesters were equipped with a tap connected to an external tube, which enabled the sampling of the exhausted biogas. These digesters were placed outdoor under direct solar radiation for 15 days, after which the produced biogas was characterized by its CH4 content (vol %) through gas chromatography. The results are reported in Table 1. In this table, it can be seen that the methanation capacity is best in the mixture containing 40 wt% catalytically pretreated coffee pulp, 40 wt% cattle manure, and 20 wt% water. The preliminary experiments described above permitted to determine the optimum coffee pulp/cattle manure ratio, assuring the biological processes during anaerobic codigestion take place while utilizing photocatalytically pretreated coffee pulp as nutrient matter.
| Pretreated coffee pulp/cattle manure | Methane concentration (%) |
|---|---|
| 0/1.0 | 3 |
| 0.5/1.0 | 7 |
| 1.0/1.0 | 15 |
| 2.0/1.0 | 6 |
| 1.0/0 | 2.5 |
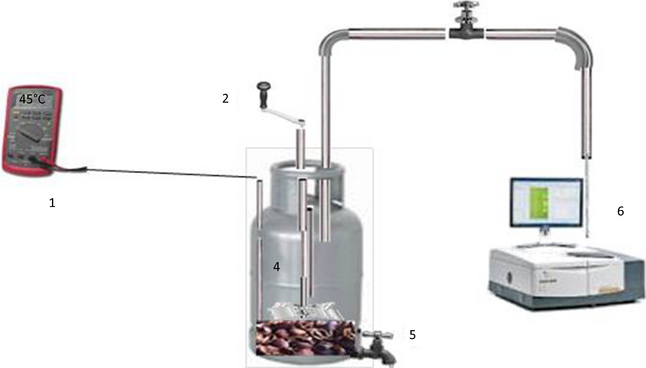
Batch digestion tests were performed using 2 L capacity stainless steel tanks as digestion reactors. Two digesters, one containing the mixture of cattle manure and photocatalytically pretreated coffee pulp (called digester A) and another (called digester B) containing the mixture of cattle manure and coffee pulp pretreated without the photocatalyst were prepared for the codigestion tests. The digesters were not pretreated with any methanogenic inoculum. A typical setup of the codigestion experiment using such digester is presented schematically in Figure 2. The mixtures contained 40 wt% pretreated or untreated coffee pulp, 40 wt% cattle manure, and 20 wt% water. After pouring the mixtures, the digesters were tightly closed with rubber septum, and left outdoor under direct solar radiation (for heating) for 30 days. It is important to note that the stainless steel tanks do not permit the solar radiation to pass through the reaction mixture. Therefore, no photocatalytic process takes place inside the tank, even if the photocatalyst in traces remains in the reaction mixture. Under these circumstances, there would be no generation of oxidizing species such as surface-bound hydroxyl radical (·OH), superoxide (·O2−), hydroperoxy radical (HO2·), and free holes, and thus the growth of anaerobic bacteria remains unaffected.
|
|
|
Figure 2. Schematic illustration of the used codigestion setup (1): thermocouple; (2): mixing crank handle; (3): activated carbon; (4): digestion reactor; (5): sampling valve for pH measurement; (6): FTIR spectrometer. |
As in the case of photocatalytic pretreatment, the average illumination intensity was about 1000 W m−2, and the maximum temperature of the reaction mixture varied in between 35 and 45°C. To provide mixing of the reactants in the containers, the reactors were agitated manually with a crank handle for about 1 min twice a day.
Analytical methods
The contents of cellulose, hemicellulose, and lignin of the coffee pulp were analyzed every 5 days for 30 days of photocatalytic pretreatment, following the method proposed by Van Soest et al. [48]. The values of the pH, total solids (TS), and the volatile solids (VS) in the reactors were determined before (initial values) and after 30 days of codigestion (final values), according to the Mexican standard method [49]. The initial and final parameters are reported in Table 2.
| In fresh coffee pulp (%) | In the product of reactor A (%) | In the product of reactor B (%) | |
|---|---|---|---|
| Lignin | 6.88 | 4.05 | 6.88 |
| Cellulose | 43.98 | 29.45 | 43.56 |
| Hemicellulose | 27.80 | 11.86 | 27.74 |
After 30 days of codigestion, the generated biogas (100 mL min−1) was passed through an activated carbon column and collected into an analysis gas cell coupled to a Bruker (Vertex 70) FTIR gas spectrometer for composition analysis. The analysis of the biogas composition was performed using the QAsoft-quantitative analysis software to determine the presence and quantity of the volatile compounds in the flow. All the measurements were performed in triplicate at room temperature.
Results and Discussion
Catalyst characterization
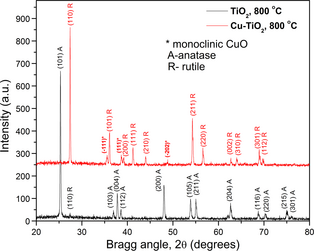
Figure 3 shows the XRD patterns of TiO2, and 10%Cu/TiO2 samples calcined at 800°C for 4 h. While the pure TiO2 sample revealed its crystallinity in anatase phase, the annealed 10%Cu/TiO2 sample reveled its rutile phase without considerable change in crystallinity. The sample also revealed a weak diffraction peak associated with CuO in monoclinic phase. Appearance of CuO peak in the 10%Cu/TiO2 composite indicates the presence of Cu dispersed in TiO2 powder. It must be noted that all the peaks associated with rutile phase of TiO2 in 10%Cu/TiO2 sample suffered a small shift toward lower angle, demonstrating a lattice expansion due to Cu incorporation.
|
|
|
Figure 3. XRD patterns of powder TiO2 and 10%Cu/TiO2 samples after calcination in air at 800°C for 4 h. For clarity, the XRD pattern of the 10%Cu/TiO2 samples was vertically shifted. XRD, X-ray diffraction. |
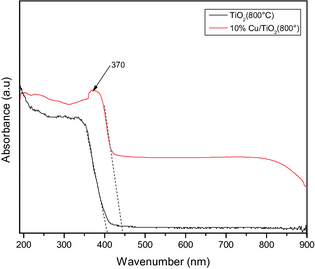
In Figure 4, the UV–Vis absorption spectra of the TiO2 and 10%Cu/TiO2 samples are presented. Absorption spectrum of the 10%Cu/TiO2 sample revealed three clearly distinguishable absorption regions. In the first region in between 220 and 310 nm, there appeared a broad absorption signal peaked at about 235 nm, which can be assigned to the charge-transfer transition of the ligand O2−, to isolated metal center Cu2+ and the d–d transition of CuO particles [50-52].
|
|
|
Figure 4. UV–Vis absorbance spectra of TiO2 and 10%Cu/TiO2 samples after calcination at 800°C for 4 h in air. |
The broad absorption signal in the 350–400 nm spectral range, peaked around 370 nm indicates the presence of Cu2O layers on the particle surface [51]. The absorption in the visible region (400–800 nm) can be attributed to the absorption of Cu2O and CuO on the TiO2 surface. On the other hand, the absorption spectrum of the pure TiO2 sample is featureless, except a strong absorption edge near about 385 nm. The band gap calculated by extrapolating the absorbance near the absorption edge to zero revealed the band gap energy values of about 3.04 and 2.77 eV for the pure TiO2 and 10%Cu/TiO2 samples, respectively. The estimated band gap energy values clearly indicate a red shift (toward visible region) of band gap energy of TiO2 on Cu incorporation. The BET estimated specific surface area and optical band gap energy values of the pure and Cu incorporated calcined TiO2 samples are presented in Table 3.
| Sample | Specific surface area (m2 g−1) | Band gap energy, Eg (eV) |
|---|---|---|
| TiO2 | 10.02 | 3.04 |
| 10%Cu/TiO2 | 12.10 | 2.77 |
Photocatalytic pretreatment of coffee pulp
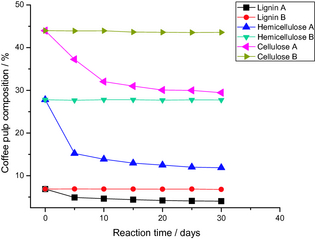
Figure 5 shows the evolution of lignin, cellulose, and hemicellulose contents present in the fresh coffee pulp, as a function of the reaction time in reactor A (with 10%Cu/TiO2 photocatalyst), and in reactor B (without the photocatalyst). Table 2 shows the estimated values of lignin, cellulose, and, hemicellulose present in the fresh coffee pulp after 30 days of photocatalytic pretreatment in reactor A, and in reactor B. From the data presented in Figure 5 and Table 2, we can conclude that:
- The contents of lignin, cellulose, and hemicellulose remain almost same on irradiating the pulp in reactor B (in absence of the photocatalyst).
- After the photocatalytic pretreatment, the contents of lignin, cellulose, and hemicellulose in the coffee pulp decreased considerably.
- Lignin, cellulose, and hemicelluloses photodegradation rate is high during the first 10 days of solar irradiation pretreatment. After this time, only a small variation in the contents of these components in reactor A was detected. This result suggests that for application convenience, coffee pulps photocatalytic pretreatment time can be reduced.
|
|
|
Figure 5. Evolution of the composition of coffee pulp as a function of reaction time. |
The results obtained from the pretreatments in two reactors (reactor A and reactor B) clearly indicate that the pretreatment without the photocatalyst has negligible effect on the degradation of lignocellulosic biomass in comparison with the pretreatment in presence of 10% Cu/TiO2 photocatalyst. As the UV–Vis absorption spectrum of the 10% Cu/TiO2 catalyst (Figure 4) revealed the presence of Cu2+ and Cu1+ ions and an extended TiO2 absorption in the visible region, the photocatalytic degradation of lignocellulosic biomass can be explained considering the following facts:
- The 10%Cu/TiO2 sample acts as a very efficient photocatalyst under solar irradiation due to its characteristic absorptions near UV region and extended absorption in the visible spectral region.
- Electron trapping by the copper ions present in the TiO2 matrix probably inhibits the recombination process of photogenerated electrons and holes [53-56].
- The 10%Cu/TiO2 photocatalyst probably generated a large amount of hydroxyl radicals (·OH), superoxides (·O2−), hydroperoxy radicals (HO2·), or free holes on its surface as proposed by Linsebigler et al. [45]. These species should have enhanced the lignocellulose degradation rate in a similar way as proposed by Machado et al. [40]. These authors found that the hydroxylation of aromatic structures in lignin occurs during photochemical process in the presence of TiO2, which is known to generate hydroxyl radicals under UV–Vis irradiation. The incorporation of hydroxyl groups into the benzene rings leads to the creation of radical sites allowing the action of superoxide radicals, and resulting in a structural fragmentation of lignin. On the other hand, Ma et al. have observed a rapid photocatalytic lignin degradation using TiO2 and Pt/TiO2 [45].
Our results indicate that a photocatalytic pretreatment might be an effective way for breaking the complex lignocellulosic biomass matrix, changing chemical components and physical structures of biomass like coffee pulp. As the lignocellulosic biomasses are very difficult to be degraded biologically, a photocatalytic pretreatment of biomass can generate biodegradable components, which would be easier to digest for microorganisms.
Anaerobic codigestion process
In order to investigate the effect of photocatalytic pretreatment on the subsequent biogas production, the coffee pulps pretreated in the reactors A and B were anaerobically codigested with cattle manure.
As can be seen from Table 4, the initial and final pH values measured for the two digesters remained constant, whether the coffee pulp was photocatalytically pretreated or not. The percentage decrease in TS and VS in the mixtures of the two digesters after 30 days of solar irradiation is considerably notable. The decrease is huge for the digester A where we sued the photocatalytically pretreated coffee pulp in comparison with the same in the digester B fed with untreated coffee pulp for the codigestion by cattle manure.
| Digester | pH | Total solids (%) | Volatile solids (%) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| A | 7.1 | 7.1 | 14.26 | 5.45 | 92.25 | 10.86 |
| B | 7.2 | 7.0 | 14.02 | 10.15 | 91.09 | 89.03 |
These results indicate that the effect of photocatalytic pretreatment of coffee pulp results in an enhanced degradation of TS and VS through the codigestion process. The result of the codigestion process is seen to be closely related with the results of photocatalytic pretreatment (Table 2), suggesting a possible correlation between the amount of degraded lignin, cellulose, and hemicelluloses in the fed charge of the digester and the produced biogas.
Biogas production
In Table 5, the results of quantitative analysis of the produced biogas in the digesters A and B are presented in comparative manner. From the obtained results, we can observe that after only 1 month of codigestion: (1) valuable combustion gases like methane, propane, etc. are generated from the coffee pulp–cattle manure mixture through codigestion; (2) the amounts of generated combustion gases are considerably higher for the digester containing photocatalytically pretreated coffee pulp (digester A) than the amounts generated from the digester B, where untreated coffee pulp was used.
| Compound | Estimated volume (mL/100 mL of biogas) | |
|---|---|---|
| Digester A | Digester B | |
| Butane | 3.26 | 1.13 |
| 1-Butene | 0.00 | 0.26 |
| Cis-2-butene | 0.00 | 1.15 |
| Cyclopropane | 6.41 | 2.17 |
| Ethane | 0.06 | 0.00 |
| Isobutane | 2.38 | 0.71 |
| Methane | 21.09 | 17.79 |
| Propane | 23.78 | 8.44 |
| 2-Methyl-1-butene | 0.00 | 2.08 |
| 3-Methyl-1-butene | 7.56 | 2.51 |
| 2-Pentene | 10.61 | 1.97 |
| Cyclohexane | 0.48 | 0.07 |
| Cyclohexene | 0.05 | 0.01 |
| Dodecane | 1.03 | 0.26 |
| n-Hexane | 6.85 | 0.35 |
| 1-Hexene | 6.83 | 2.07 |
| 3-Methyl pentane | 2.62 | 0.92 |
| n-Octane | 0.05 | 0.22 |
| Carbon dioxide | 11.03 | 43.25 |
| Hydrogen sulfide | 2.06 | 0.92 |
| Other gases | 5.68 | 15.79 |
| Total | 100 | 100 |
Table 6 shows the combustion enthalpies (ΔHc) of the combustion gases detected in 100 mL of the biogas generated in digesters A and B. These values were calculated from the volume (V) of each component measured in 100 mL of biogas (Table 5) and on basis of the standard combustion enthalpies ( ) reported in the literature using the following relation[57]:
|
|
| Compound | kcal mol−1 | ∆Hc kcal/100 mL biogas | |
|---|---|---|---|
| Digester A | Digester B | ||
| Butane | −687.982 | −0.10 | −0.03 |
| 1-Butene | −649.757 | 0 | 0 |
| Cis-2-butene | −648.115 | 0 | −0.03 |
| Cyclopropane | −499.52 | −0.14 | −0.04 |
| Ethane | −372.820 | 0 | 0 |
| Isobutane | −686.342 | −0.07 | −0.02 |
| Methane | −212.798 | −0.20 | −0.17 |
| Propane | −530.605 | −0.56 | −0.19 |
| 2-Methyl-1-butene | −803.17 | 0 | −0.07 |
| 3-Methyl-1-butene | −804.93 | −0.27 | −0.09 |
| 2-Pentene | −805.34 | −0.38 | −0.07 |
| Cyclohexane | −944.79 | −0.02 | −0.003 |
| Cyclohexene | −907.38 | −0.00 | −0.001 |
| Dodecane | −1947.23 | −0.09 | −0.02 |
| n-Hexane | −1002.57 | −0.30 | −0.01 |
| 1-Hexene | −973.42 | −0.29 | −0.08 |
| 3-Methyl pentane | −1001.51 | −0.11 | −0.04 |
| n-Octane | −1317.45 | −0.002 | −0.01 |
| Total | −2.59 | −0.93 | |
As can be seen from the Table 6, the total combustion enthalpy (ΔHc) of the biogas generated in digester A is almost three times to the total combustion enthalpy of the biogas generated in the digester B ([−2.59]/[−0.93] = 2.78), indicating the necessity of utilizing the photocatalytic pretreatment of coffee pulp before its digestion for obtaining higher amounts of biogas, that is, for higher economic benefits. The equivalent heat of combustion of the biogas generated in digester A is approximately 2427 kJ/mol, which is much higher than the heat of combustion of methane (889 kJ/mol), the principal component of usual biogas.
The results presented in the Tables 2 and 4-6 suggest that a photocatalytic pretreatment of the coffee pulp before the codigestion process might have accelerated its subsequent anaerobic biodegradation.
Anaerobic degradation of biomass is considered to follow a sequence of four steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. During the hydrolysis phase, the water-insoluble compounds like cellulose, proteins, and fats get broken into monomers (water-soluble fragments) by exoenzimes (hydrolase). The conversions of carbohydrates into simple sugars, lipids into fatty acids, and proteins into amino acids take place in the hydrolysis phase [58]. However, the degradation of lignocellulose and lignin present in biomass is slow and incomplete [59].
As we can see from Table 2, after the photocatalytic pretreatment, the contents of lignin, hemicelluloses, and cellulose in the coffee pulp decrease, probably due to the cleavage of chemical bonds of these components during the photocatalytic pretreatment process. The presence of lignin in lignocellulosic biomass leads to a protective barrier to the biomass and provides resistance to any chemical and biological degradation, preventing the plant cell destruction by fungi, bacteria, or enzymes. For the generation of biogas from coffee pulp through codigestion, the cellulose and hemicellulose must be broken to their corresponding monomers, suitable for their digestions through biological route (by microorganisms). Therefore, the photocatalytic pretreatment of coffee pulp with 10%Cu/TiO2 under solar radiation in presence of oxygen helps to cleavage the impermeable/resistant layer of lignin, and breaks the containing long-chain cellulose and hemicellulose into their corresponding monomers. The enhanced codigestion of the photocatalytically pretreated coffee pulp and cattle manure mixture is due to the presence of a reduced amount of lignin and increased availability of lignocellulosic monomers in the pretreated coffee pulp.
It is worth noting (Tables 5 and 6) that besides methane, high amounts of propane, butane, and other high molecular weight combustible hydrocarbons are generated during the codigestion of photocatalytically pretreated coffee waste with cattle manure. The presence of these high molecular weight products could be due to the following factors:
- The variations in temperature inside the digester.
- An inhibition effect due to traces of 10%Cu/TiO2 (remaining from the photocatalytic pretreatment) in the anaerobic digester.
- The presence of additional products generated from the photocatalytic hydroxylation of the aromatic structures of lignin, hemicelluloses, and cellulose.
As the temperature variation was similar inside both the digesters (A and B), the contribution of the first factor can be discarded. However, the composition of the biogas generated from digester A is different from the digester B. The contribution of the second factor should also be ruled out, as the used photocatalyst (10% Cu/TiO2) is not active inside the stainless steel digester (as discussed in the section Anaerobic codigestion tests). Now, it is possible that during the photocatalytic pretreatment of lignin, hemicelluloses, and cellulose, several derivatives of their aromatic structures are produced in high concentration through photocatalytic hydroxylation, which might also act as bacterial nutrients. The excessive increase in bacterial nutrients might also cause a reduction in consumption of higher carbon content organic molecules by the anaerobic bacteria in the digestion process.
The results presented in Table 6 indicate that the biogas produced by our process can generate higher amount of energy during its combustion than the conventional biogases rich in methane. However, we are still optimizing the catalysis process and expecting even a higher biogas output. The optimized yield and the viability data of the process will be communicated soon.
Conclusions
We have demonstrated that a photocatalytic pretreatment of waste coffee pulp using 10%Cu/TiO2 photocatalyst under solar radiation and air as oxidizing agent can enhance the solubility and biodegradability of its lignocellulosic components. This improved biodegradability also enhances the production of biogas from this waste biomass through anaerobic digestion. The process described in this study is technically viable, produces biogas of high combustion energy, and thus promising for higher economic benefits. Moreover, the process has potential for larger-scale production of energy-rich biogas from natural wastes like coffee pulp.
Acknowledgments
The authors acknowledge VIEP & DITCo, BUAP (Grants # VIEP 45/NAT/2014, DITCo-BUAP/INOVA/3 and DITCo-BUAP/INOVA/19), and CONACyT, Mexico (Grant # CB-2010/151767) for their financial supports.
Conflict of Interest
None declared.
References
- Simpson-Holley, M., A. Higson, and G. Evans. 2007. Bring on the biorefinery. J. Chem. Eng.163:46–59.
- Fantozzi, F., and C. Buratti. 2009. Biogas production from different substrates in an experimental continuously stirred tank reactor anaerobic digester. Bioresour. Technol.100:5783–5789.
- Abbasi, T., and S. A. Abbasi. 2010. Biomass energy and the environmental impacts associated with its utilization. Renew. Sustain. Energy Rev.14:919–937.
- Fantozzi, F., and C. Buratti. 2011. Anaerobic digestion of mechanically treated OFMSW: experimental data on biogas/methane production and residues characterization. Bioresour. Technol.102:8885–8892.
- Boullaqui, H., Y. Touhami, R. B. Cheikh, and M. Hamdi. 2005. Bioreactor performance in anaerobic digestion of fruit and vegetables waste. Process Biochem.40:989–995.
- Gallert, C., A. Henning, and J. Winter. 2003. Scale-up of anaerobic digestion of the biowaste fraction from domestic wastes. Water Resour.37:1433–1441.
- Abarca-Guerrero, L., G. Maas, and W. Hogland. 2013. Solid waste management challenges for cities in developing countries. Waste Manag.33:220–232.
- Lopes, W. S., V. D. Leite, and S. Prasad. 2004. Influence of inoculum on performance of anaerobic reactors for treating municipal solid waste. Bioresour. Technol.94:261–266.
- Brand, D., A. Pandey, S. Roussos, and C. R. Soccol. 2000. Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme Microb. Technol.26:127–130.
- Pandey, A., C. R. Soccol, P. Nigam, D. Brand, R. Mohan, and S. Roussos. 2000. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. J. Biochem. Eng6:153–158.
- Deublein, D., and A. Steinhauser. 2008. Biogas from waste and renewable sources: an introduction. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
- Mussatto, S. I., S. E. M. Ercilla, S. Martins, and A. T. Jose. 2011. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol.4:661–672.
- Franca, A. S., I. S. Oliviera, and M. E. Ferreira. 2009. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination249:267–272.
- Murthy, P. S., and M. M. Naidu. 2012. Resources. Conservation and Recycling66:45–58.
- Adams, M. R., and J. Dougan. 1981. Biological management of coffee processing wastes. Trop. Sci.23:177–185.
- Boopathy, R., and M. Mariappan. 1984. Coffee pulp: a potential source of energy. J. Coffee Res.14:108–110.
- Boopathy, R., M. Mariappan, and B. B. Sunderasan. 1986. The carbon to nitrogen ratio and methane production of coffee pulp. J. Coffee Res.16:47–50.
- Fan, L., A. T. Soccol, A. Pandey, and C. R. Soccol. 2003. Cultivation of pleurotus mushroom on Brazilian coffee husk and its effect on caffeine and tannic acid. Micologia Aplicada International15:15–21.
- Belgiorno, V., D. Panza, L. Russo, V. Amodio, and A. Cesaro. 2011. Alternative stabilization options of mechanically sorted organic fraction from municipal solid wastes prior to landfill disposal. Int. J. Environ. Eng.3:318–335.
- Neves, L., R. Oliveira, and M. M. Alves. 2006. Anaerobic co-digestion of coffee waste and sewage sludge. Waste Manag.26:176–181.
- Li, Y. Y., and T. Noike. 1992. Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment. Water Sci. Technol.26:857–866.
- Climent, M., I. Ferrer, M. Baeza, A. Artola, F. Vazquez, and X. Font. 2007. Effects of thermal and mechanical pretreatments on secondary sludge on biogas production under thermophylic conditions. Chem. Eng. J.133:335–342.
- Komenmoto, K., Y. G. Lim, Y. Onoue, C. Niwa, and T. Toda. 2007. Effects of temperature on VFAs and biogas production in anaerobic solubilization of food waste. Waste Manag.29:2950–2955.
- Heo, N., S. Park, and H. Kang. 2009. Solubilization of waste activated sludge by alkaline pretreatment and biochemical methane potential (BMP) test for anaerobic codigestion of municipal organic waste. Water Sci. Technol.48:1505–1509.
- Zhang, G., J. Yang, H. Liu, and J. Zhang. 2009. Sludge ozonation: disintegration, supernatant changes and mechanisms. Bioresour. Technol.100:1505–1509.
- Masse, L., D. I. Masse, and K. J. Kennedy. 2003. Effect of hydrolysis pretreatment on fat degradation during anaerobic digestion of slaughterhouse wastewater. Process Biochem.38:1365–1372.
- Mendes, A. A., E. B. Pereira, and H. F. de Castro. 2006. Effect of enzymatic hydrolysis of lipids-rich wastewater on the anaerobic biodigestion. Biochem. Eng. J.32:185–190.
- Xu, G., S. Chen, J. Shi, S. Wang, and G. Zhu. 2010. Combination of ultrasound and ozone for improving solubilization and anaerobic biodegradability of waste activated sludge. J. Hazard. Mater.180:340–346.
- Fdez-Guelfo, L., C. Alvarez-Gallego, B. Sales, and L. I. Romero. 2011. The use of thermochemical and biological pretreatments to enhance organic matter hydrolysis and solubilization from organic fraction of municipal solid waste. Chem. Eng. J.168:249–254.
- Liu, J., Y. Wu, and N. Xu. 1995. Effects of ammonia bicarbonate treatment on kinetics of fiber digestion, nutrient digestibility and nitrogen utilization of rice straw by sheep. Anim. Feed Sci. Technol.52:131–139.
- Patel, M. M., and R. M. Bhatt. 1992. Optimization of the alkaline peroxide pretreatment for the delignification of rice straw and its applications. J. Chem. Technol. Biotechnol.53:253–263.
- Sankat, C., and B. Lauckner. 1991. The effect of ammonia treatment on the digestibility of rice straw under various process conditions. Can. Agr. Eng.33:309–314.
- Driscoll, M., A. Stipanovic, W. Winter, K. Cheng, M. Manning, J. Spese, et al. 2009. Electron beam irradiation of cellulose. Radiat. Phys. Chem.78:539–542.
- Ng, F. M. F., and K. N. Yu. 2007. X-ray irradiation induced degradation of cellulose nitrate. Mater. Chem. Phys.100:38–40.
- Discoll, M., A. Stimanovic, W. Winter, K. Cheng, M. Manning, J. Spiese, et al. 2009. Electron beam irradiation of cellulose. Radiat. Phys. Chem.78:539–542.
- Ghanbari, F., T. Ghoorchi, P. Shawrang, H. Monsouri, and N. M. Torbati-Nejad. 2012. Comparison of electron beam and gamma ray irradiations effects on ruminal crude protein and amino acid degradation kinetics and in vitro digestibility of cottonseed meal. Radiat. Phys. Chem.81:672–678.
- Uyguner, C. S., and M. Bekbolet. 2010. TiO2 assisted photocatalytic degradation of humic acids: effect of copper ions. Water Sci. Technol.61:2581–2590.
- Machado, A. E. H., A. M. Furuyama, S. Z. Falone, R. Ruggerio, D. S. Perez, and A. Castellan. 2000. Photocatalytic degradation of lignin and lignin models, using titanium dioxide: the role of the hydroxyl radical. Chemosphere40:115–124.
- Selli, E., D. Baglio, L. Montanarella, and G. Bidoglio. 1999. Role of humic acids in the TiO2-photocatalyzed degradation of tetrachloroethene in water. Water Resour.33:1827–1936.
- Tanaka, K., C. R. Calanag, and T. Hisanaga. 1999. Photocatalyzed degradation of lignin on TiO2 . J Mol. Catal. A Chem.38:287–294.
- Dunn, W. W., Y. Aikawa, and A. J. Bard. 1981. Characterization of particulate titanium dioxide photocatalysts by photoelectrophoretic and electrochemical measurements. J. Am. Chem. Soc.100:3456–3459.
- Peterson, M. W., J. A. Turner, and A. J. Nozic. 1991. Mechanistic studies of the photocatalytic behavior of TiO2. Particles in a photoelectrochemical slurry cell and the relevance of photodetoxification reactions. J. Phys. Chem.95:221–225.
- Fox, M. A., and M. T. Dulay. 1993. Heterogeneous photocatalysis. Chem. Rev.93:341–357.
- Linsebigler, A. L., G. Lu, and J. T. Yates Jr. 1995. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev.95:735–741.
- Ma, Y. S., C. N. Chang, Y. P. Chiang, H. F. Sung, and A. C. Chao. 2008. Photocatalytic degradation of lignin using Pt/TiO2 as the catalyst. Chemosphere71:998–1004.
- Gaya, U. I., and A. H. Abdullah. 2010. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol.C-9:1–12.
- Huang, G. L., S. C. Zhang, T. G. Xu, and Y. F. Zhu. 2008. Fluorination of ZnWO4 photocatalyst and influence on the degradation mechanism for 4-chlorophenol. Environ. Sci. Technol.42:8516–8521.
- Van Soest, P., J. Robertson, and B. Lewis. 1991. Symposium: carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. J. Dairy Sci.74:3583–3597.
- Mexican Official Standard: NOM-AA-34-1976, ISO 14000.
- Cordoba, G., R. Arroyo, J. L. G. Fierro, and M. Viniegra. 1996. Study of Xerogel-Glass Transition of CuO/SiO2 . J. Solid State Chem.123:93–98.
- Boccuzzi, F., S. Coluccia, G. Martra, and N. Ravasio. 1999. Cu/SiO2 and Cu/SiO-2TiO2 catalysts: 1. TEM, DR UV–vis-NIR, and FTIR characterization. J. Catal.184:316–322.
- Velu, S., K. Suzyki, M. Akazaki, M. P. Kapoor, T. Osaki, and F. Ahashi. 2000. Oxidative steam reforming of methanol over CuZnAl(Zr)-oxide catalysts for the selective production of hydrogen for fuel cells: catalyst characterization and performance evaluation. J. Catal.194:373–379.
- Xin, B., P. Wang, D. Ding, J. Liu, Z. Ren, and H. Fu. 2008. Effect of surface species on Cu-TiO2 photocatalytic activity. Appl. Surf. Sci.254:2569–2574.
- Foster, N., R. Noble, and C. Koval. 1993. Reversible photoreductive deposition and oxidative dissolution of copper ions in titanium dioxide, aqueous suspensions. Environ. Sci. Technol.27:350–356.
- Ward, M., and A. Bard. 1982. Photocurrent enhancement via trapping of photogenerated electrons of TiO, particles. J. Phys. Chem.86:3599–3605.
- Butler, E. C., and A. P. Davis. 1993. Photocatalytic oxidation in aqueous titanium dioxide suspensions: the influence of dissolved transition metals. J. Photochem. Photobiol., A70:273–283.
- Perry RH, R. H. and D. W. Gree. 1999. Perrys Chemical Engineers' handbook. Mc Graw Hill Companies Inc, McGraw Hill, New York, USA.
- Gerardi, M. H.2003. The microbiology of anaerobic digesters, waste water microbiology series. John Wiley and Sons Inc, Hoboken, NJ.
- Deublein, D., and A. Steinhauser. 2008. Biogas from waste and renewable sources: an introduction. WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.
References
- Simpson-Holley, M., A. Higson, and G. Evans. 2007. Bring on the biorefinery. J. Chem. Eng.163:46–59.
- Fantozzi, F., and C. Buratti. 2009. Biogas production from different substrates in an experimental continuously stirred tank reactor anaerobic digester. Bioresour. Technol.100:5783–5789.
- Abbasi, T., and S. A. Abbasi. 2010. Biomass energy and the environmental impacts associated with its utilization. Renew. Sustain. Energy Rev.14:919–937.
- Fantozzi, F., and C. Buratti. 2011. Anaerobic digestion of mechanically treated OFMSW: experimental data on biogas/methane production and residues characterization. Bioresour. Technol.102:8885–8892.
- Boullaqui, H., Y. Touhami, R. B. Cheikh, and M. Hamdi. 2005. Bioreactor performance in anaerobic digestion of fruit and vegetables waste. Process Biochem.40:989–995.
- Gallert, C., A. Henning, and J. Winter. 2003. Scale-up of anaerobic digestion of the biowaste fraction from domestic wastes. Water Resour.37:1433–1441.
- Abarca-Guerrero, L., G. Maas, and W. Hogland. 2013. Solid waste management challenges for cities in developing countries. Waste Manag.33:220–232.
- Lopes, W. S., V. D. Leite, and S. Prasad. 2004. Influence of inoculum on performance of anaerobic reactors for treating municipal solid waste. Bioresour. Technol.94:261–266.
- Brand, D., A. Pandey, S. Roussos, and C. R. Soccol. 2000. Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme Microb. Technol.26:127–130.
- Pandey, A., C. R. Soccol, P. Nigam, D. Brand, R. Mohan, and S. Roussos. 2000. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. J. Biochem. Eng6:153–158.
- Deublein, D., and A. Steinhauser. 2008. Biogas from waste and renewable sources: an introduction. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
- Mussatto, S. I., S. E. M. Ercilla, S. Martins, and A. T. Jose. 2011. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol.4:661–672.
- Franca, A. S., I. S. Oliviera, and M. E. Ferreira. 2009. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination249:267–272.
- Murthy, P. S., and M. M. Naidu. 2012. Resources. Conservation and Recycling66:45–58.
- Adams, M. R., and J. Dougan. 1981. Biological management of coffee processing wastes. Trop. Sci.23:177–185.
- Boopathy, R., and M. Mariappan. 1984. Coffee pulp: a potential source of energy. J. Coffee Res.14:108–110.
- Boopathy, R., M. Mariappan, and B. B. Sunderasan. 1986. The carbon to nitrogen ratio and methane production of coffee pulp. J. Coffee Res.16:47–50.
- Fan, L., A. T. Soccol, A. Pandey, and C. R. Soccol. 2003. Cultivation of pleurotus mushroom on Brazilian coffee husk and its effect on caffeine and tannic acid. Micologia Aplicada International15:15–21.
- Belgiorno, V., D. Panza, L. Russo, V. Amodio, and A. Cesaro. 2011. Alternative stabilization options of mechanically sorted organic fraction from municipal solid wastes prior to landfill disposal. Int. J. Environ. Eng.3:318–335.
- Neves, L., R. Oliveira, and M. M. Alves. 2006. Anaerobic co-digestion of coffee waste and sewage sludge. Waste Manag.26:176–181.
- Li, Y. Y., and T. Noike. 1992. Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment. Water Sci. Technol.26:857–866.
- Climent, M., I. Ferrer, M. Baeza, A. Artola, F. Vazquez, and X. Font. 2007. Effects of thermal and mechanical pretreatments on secondary sludge on biogas production under thermophylic conditions. Chem. Eng. J.133:335–342.
- Komenmoto, K., Y. G. Lim, Y. Onoue, C. Niwa, and T. Toda. 2007. Effects of temperature on VFAs and biogas production in anaerobic solubilization of food waste. Waste Manag.29:2950–2955.
- Heo, N., S. Park, and H. Kang. 2009. Solubilization of waste activated sludge by alkaline pretreatment and biochemical methane potential (BMP) test for anaerobic codigestion of municipal organic waste. Water Sci. Technol.48:1505–1509.
- Zhang, G., J. Yang, H. Liu, and J. Zhang. 2009. Sludge ozonation: disintegration, supernatant changes and mechanisms. Bioresour. Technol.100:1505–1509.
- Masse, L., D. I. Masse, and K. J. Kennedy. 2003. Effect of hydrolysis pretreatment on fat degradation during anaerobic digestion of slaughterhouse wastewater. Process Biochem.38:1365–1372.
- Mendes, A. A., E. B. Pereira, and H. F. de Castro. 2006. Effect of enzymatic hydrolysis of lipids-rich wastewater on the anaerobic biodigestion. Biochem. Eng. J.32:185–190.
- Xu, G., S. Chen, J. Shi, S. Wang, and G. Zhu. 2010. Combination of ultrasound and ozone for improving solubilization and anaerobic biodegradability of waste activated sludge. J. Hazard. Mater.180:340–346.
- Fdez-Guelfo, L., C. Alvarez-Gallego, B. Sales, and L. I. Romero. 2011. The use of thermochemical and biological pretreatments to enhance organic matter hydrolysis and solubilization from organic fraction of municipal solid waste. Chem. Eng. J.168:249–254.
- Liu, J., Y. Wu, and N. Xu. 1995. Effects of ammonia bicarbonate treatment on kinetics of fiber digestion, nutrient digestibility and nitrogen utilization of rice straw by sheep. Anim. Feed Sci. Technol.52:131–139.
- Patel, M. M., and R. M. Bhatt. 1992. Optimization of the alkaline peroxide pretreatment for the delignification of rice straw and its applications. J. Chem. Technol. Biotechnol.53:253–263.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?