(Created page with " ==Summary== ====Background==== Laparoscopic sleeve gastrectomy (LSG) is a popular stand-alone bariatric surgery, despite a paucity of long-term data. Hence, this study is...") |
m (Scipediacontent moved page Draft Content 386888040 to Pok et al 2015a) |
(No difference)
| |
Latest revision as of 11:43, 26 May 2017
Summary
Background
Laparoscopic sleeve gastrectomy (LSG) is a popular stand-alone bariatric surgery, despite a paucity of long-term data. Hence, this study is to report the long-term outcome of LSG as primary bariatric procedure and the result of revisional surgery.
Methods
With retrospective analysis of a prospective bariatric database, participants who defaulted clinic follow-up were interviewed by telephone. A total of 667 LSG was performed as primary bariatric procedure (2006–2012) with mean age of 34.5 ± 9.7 years old, female 74.7%, mean body mass index (BMI) 37.3 ± 8.1 kg/m2. A 36-F bougie was used for all cases.
Results
There were 61 patients available with long-term data. The weight loss outcome at 1 year, 2 years, 3 years, 4 years, and 5 years showed a mean BMI 26.3, 25.2, 25.3, 27.1, and 26.2 with mean excess weight loss (EWL) 76.0%, 79.6%, 77.3%, 73.4%, and 72.6% respectively. However, 17% patients developed de novo gastro-esophageal reflux disease (GERD). Eighteen patients (2.2%) needed surgical revisions due to weight regain (n = 6), persistent type 2 diabetes mellitus (T2DM; n = 2), stricture (n = 2), and GERD (n = 8). The revision resulted in an additional mean excess weight loss of 23.8% with mean BMI 24.9 kg/m2 at 6 months postoperatively. There was a 23.7% mean reduction of HbA1c with one patient who was in complete diabetic remission at 1 year.
Conclusion
Our results showed LSG is a durable bariatric procedure with > 70% EWL at 5 years despite a high incidence of GERD. The need for revision of LSG is low and mainly for GERD.
Keywords
gastro-esophageal reflux;long-term outcome;morbid obesity;sleeve gastrectomy;revision
1. Introduction
Laparoscopic sleeve gastrectomy (LSG) has been gaining popularity as a stand-alone bariatric surgery worldwide since the first inception of this novel procedure in 1988. It was first described as the initial step, as part of biliopancreatic bypass with duodenal switch (DS) for obesity by Dr. Doug Hess1 in March 1998. In a similar development, Dr. Gagner et al2 used it as a first stage bariatric procedure for high risk patients in order to reduce the risk profile of patient, and later performed gastric bypass once adequate weight loss was achieved. However, it was noted that the sleeve gastrectomy alone could cause good weight loss before the second procedure. In fact, this finding was consistent with other published reports.3 ; 4 Due to its effectiveness in weight loss and resolution of comorbidities, it was then used as the primary restrictive bariatric procedure and verified in three International Consensus Summit of Sleeve Gastrectomy in 2007, 2009, and 2011 as a safe and feasible primary bariatric procedure.5; 6 ; 7 In fact, the American College of Surgeons Bariatric Surgery Center Network has put it in the intermediate position between laparoscopic gastric banding and laparoscopic gastric bypass in terms of reduction of body mass index (BMI), complication rates, and resolution of obesity related illness.8
However, until now, the technique of LSG is still not standardized. This is because, different bougie sizes have been used to calibrate the diameter of the sleeve and variation of technical approaches might have led to different results from this procedure. At the moment, despite the paucity of long-term outcomes, sleeve gastrectomy has gained wide acceptance by patients based on the short and medium term data. In addition, with the expanding field of bariatric surgery and misconception of laparoscopic sleeve gastrectomy as an easier operation, the complication rate and the need of revisional surgery for complicated or failed primary LSG would be expected to increase especially in the hands of inexperienced surgeons.
Therefore, the aim of this study is to report our 7 years experiences of LSG in Asia as a primary bariatric procedure regarding the short- and long-term clinical outcomes especially in the maintenance of effective weight loss. It is also necessary to know the cause of failure of LSG as well as the indications and outcomes following revisional surgery for LSG. Key variations of surgical techniques over the years is also described in our practice.
2. Materials and methods
We performed a retrospective review of patients who underwent LSG from 2006 to 2012 who have completed at least 6 months follow-up. Informed consent was taken from all patients and data were collected prospectively into a database which was later analyzed retrospectively. The baseline characteristic, surgical outcome, weight loss and comorbidity resolution, and revisional surgery were included for this analysis. Patients who had surgery > 5 years ago but defaulted follow-up, were contacted by telephone to enquire upon their weight status and reflux symptoms. A total of 667 LSG was performed as the primary bariatric procedure. The patients' mean age was 34.5 ± 9.7 years (range 14–71 years) with a female dominant cohort of 74.7%. The preoperative mean BMI was 37.3 ± 8.1 kg/m2 (range 20.8–75.3 kg/m2) with mean excess weight 41.7 ± 23.3 kg.
Effectiveness end points include BMI, percentage of excess weight loss (% EWL) from baseline to 6 months and yearly with trend up to 7 years. Safety end points were defined by the 30 days perioperative major and minor complications after surgery.
The indication for revision of LSG was examined. Failure of primary procedure is defined as inadequate weight loss (< 50% EWL) or weight regain (> 10 kg body weight), or failure of resolution of diabetes mellitus. Complicated LSG is defined as long-term side effects secondary to sleeve gastrectomy such as GERD. Surgery for treatment of perioperative complications occurring within 30 days after primary surgery were not included in the analysis for revisional surgery.
2.1. Surgical technique
Our surgical technique of sleeve gastrectomy has evolved over the years with reinforcement suture and invagination of the stapler line which was introduced in 20069 and reduced ports access surgery started in 2009 as previously reported.10 Subsequently, we used the transumbilical two site modified single incision laparoscopic surgery (SILS) technique for all LSG.
Important surgical techniques are briefly described. All procedures were completed laparoscopically. Three skin incisions were placed at two sites of the abdomen, including two skin incisions along the natural fold of umbilicus for 10-mm port for videoscope and a 12-mm port for the left working port, and one skin incision at left lateral abdominal wall for a 5-mm port as right working port. A 2-mm Kircher wire was used as liver retractor and inserted through a subxiphoid skin puncture for exposition of the angle of His. The greater omentum and short gastric arteries were dissected by using 5-mm blunt tip laparoscopic Ligasure (Covidien, Norwalk, CT, USA) system, starting at 4 cm from pylorus (2nd branch of right gastro-epiploic artery) to the angle of His sparing the sling fibers near cardio-esophageal junction and gastro-epiploic vessels. A large fat pad at an angle of His was dissected to provide a clean field for gastric fundus resection. Meticulous dissection was performed at the angle of His with full mobilization of the gastric fundus. The posterior wall of the stomach was freed from the pancreatic adhesion if present. Once the stomach was completely mobilized, an oro-gastric tube size 36 F was placed along the lesser curvature of stomach directed toward the pylorus as a calibrator before starting the gastric resection. Vertical transection of the stomach was accomplished with five to six firings of a 60-mm linear stapler (Endo GIA, Covidien, Norwalk, CT, USA). The firing stapler height was determined by thickness of the gastric tissue, using a green (4.1 mm) or black (4.4 mm) stapler near the antrum and a blue (3.5 mm) stapler for the rest of the gastric resection. The important technical details during the gastric transection includes symmetrical lateral traction of the stomach to ensure an equal proportion of the anterior and posterior walls were included in each firing of the staplers; avoiding a spiral stapler line and minimal 1-cm margin of gastric fundus from the angle of His to avoid a critical vascular area in the cardio-esophageal junction. After gastric resection, the long gastric remnant stapler line was invaginated with a 3-0 vicryl suture to prevent leakage and hemorrhage. However, with the introduction of our technique of sleeve gastrectomy and gastric plication, a nonabsorbable suture was used instead for plication of the stapler line with a 36-F bougie inside. The gastric tube was then fixed to the posterior peritoneal tissue to prevent gastric volvulus. No intraoperative leak test was employed and no drain was used routinely. The resected stomach was extracted through the umbilical incision after dilatation without using endobag. The fascial defect was closed with a vicryl suture or plugged with surgicel (Surgicel® Hemostat (Johnson & Johnson, Arlington, TX)).11
3. Results
3.1. Outcome of primary sleeve gastrectomy
3.1.1. Surgical outcome
The mean surgical time for LSG was 117 ± 34.5 minutes with median blood loss of 30 mL. The average length of postoperative stay was 3.5 ± 3.4 days. The overall postoperative 30 days morbidity rate was minor 5.5% (n = 37) and major 3.1% (n = 20) respectively as showed in Table 1. Fifteen cases needed reoperation due to leakage (n = 11), major bleeding (n = 1), bowel injury (n = 2), and port site hernia (n = 1). There was one patient who underwent LSG in 2006 during the learning curve developed gastric tube stricture which resolved with endoscopic dilatation. There were 14 postoperative leaks detected in our large series (2.1%). However, the risk of leak was highly influenced by the learning curve of surgeons as most of the leak occurred in the first 100 cases (9%), dropped to 1–2% for the subsequent 300 cases. There was no more postoperative leakage noted for the recent 300 cases in our series (0%). For the management of leakage as shown in Table 1, three cases with minor leak healed with conservative management; 11 cases needed reoperation for wash-out and insertion of drains for drainage. However, two cases required additional endoscopic stenting due to high leakage at the cardio-esophageal junction. All leaks healed subsequently except one case of high leakage at the cardio-esophageal junction eventually needed conversion to Roux-en-Y gastric bypass (RYGB) after 6 months due to chronic fistula. There was only one mortality (0.13%) due to undiagnosed obstructive sleep apnea. The patient developed respiratory failure immediately after surgery which required ventilation in intensive care unit (ICU) but died on the same day.
| Parameter | Mean ± SD/n | Management |
|---|---|---|
| Surgical duration (min) | 117 ± 34.5 | |
| Estimated blood loss (mL) | 30a | |
| Postoperative stay (d) | 3.5 ± 3.4 | |
| Time to flatus (d) | 1.7 ± 0.6 | |
| Perioperative complication | ||
| Major | ||
| Leakage | 14 | 11 reoperation with drain insertion, 2 required stenting to due high leak, 1 converted to RYGB |
| Major bleeding | 1 | Reoperation due to intraabdominal bleeding |
| Stricture | 1 | Endoscopic dilatation |
| Bowel injury | 2 | 1 small bowel injury with repair, 1 colon injury required colectomy |
| Cardiac failure | 1 | Medical care |
| Port site hernia | 1 | Reoperation |
| Minor | ||
| Abdominal wall abscess | 1 | Wound care |
| Subcutaneous hematoma | 3 | Wound care |
| Wound infection | 17 | Wound care |
| Minor bleeding | 15 | Blood transfusion |
| Pneumonia | 1 | Medical care |
RYGB = Roux-en-Y gastric bypass.
a. Median.
3.1.2. Weight loss and comorbidity outcome
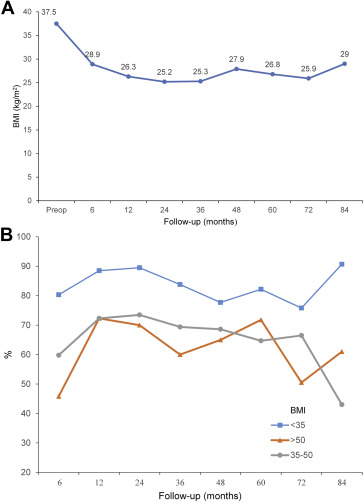
Postoperatively, the mean BMI for 6 months, 1 year, 2 years, 3 years, 4 years, and 5 years were 28.9, 26.3, 25.2, 25.3, 27.9, and 26.2, with a mean % EWL of 64.2%, 76.0%, 79.6%, 77.3%, 73.4%, and 71.6% respectively (Table 2). The evolution of overall BMI change is depicted in Fig. 1A. However, the effect of LSG was more pronounced in lower BMI patients (< 35 kg/m2) throughout a 5 year period. In 5 years, the % EWL was up to 80% for BMI < 35 kg/m2, but it was slightly lower for BMI 35–50 kg/m2 and BMI > 50 kg/m2, which was 64.7% and 71.8% respectively. (Fig. 1B) The maximal weight loss was achieved within 2 years but showed small weight regain subsequently. Although the number of follow-up patients for 6 years (n = 19) and 7 years (n = 12) were lesser, the effective weight loss effect still maintain beyond 70% EWL with BMI < 30 kg/m2
| Parameter | Baseline, n = 669 | 6 mo, n = 311 | 1 y, n = 289 | 2 y, n = 138 | 3 y, n = 66 | 4 y, n = 66 | 5 y, n = 61 | 6 y, n = 19 | 7 y, n = 12 |
|---|---|---|---|---|---|---|---|---|---|
| Body weight (kg) | 100.6 ± 26.4 | 78.4 ± 19.3 | 71.7 ± 16.2 | 68.2 ± 15.3 | 68.3 ± 14.5 | 71.7 ± 17.6 | 72.4 ± 15.6 | 69.5 ± 17.2 | 80.3 ± 26.2 |
| BMI (kg/m2) | 37.5 ± 1.4 | 28.9 ± 1.1 | 26.3 ± 2.5 | 25.2 ± 3.6 | 25.3 ± 0.8 | 27.9 ± 4.3 | 26.8 ± 4.0 | 25.9 ± 1.5 | 29 ± 9.1 |
| %WL | – | 24.9 ± 7.0 | 29.8 ± 8.2 | 30.5 ± 9.2 | 27.6 ± 9.4 | 28.5 ± 10.3 | 27.8 ± 10.9 | 29.5 ± 18.3 | 25.3 ± 7.9 |
| %EWL | – | 64.2 ± 17.1 | 76.0 ± 11.5 | 79.6 ± 36.6 | 77.3 ± 11.8 | 73.4 ± 39.5 | 71.6 ± 32.7 | 71 ± 15.7 | 70 ± 14.2 |
| Waist (cm) | 102.1 ± 41.5 | 90.3 ± 15.5 | 85 ± 12.9 | 85.1 ± 12.7 | 82.7 ± 10.2 | 88.4 ± 16.5 | 83.6 ± 7.2 | — | — |
Data are presented as mean ± SD.
% EWL percentage excess weight loss; % WL = percentage weight loss; BMI = body mass index.
|
|
|
Figure 1. Weight loss progress postLSG over 7 years in different BMI groups (A) overall BMI change and (B) % EWL. % EWL = percentage excess weight loss; BMI = body mass index; LSG = laparoscopic sleeve gastrectomy. |
All obesity related comorbidities decreased significantly after surgery in 1 year. There was > 90% reduction of the prevalence of diabetes in our cohort. Conversely, 17% (5/29) of the patients developed de novo GERD in 5 years follow-up and required long-term proton-pump inhibitor.
Out of 667 patients who underwent LSG, 27 were operated in 2006, 41 in 2007; 51 in 2008; 50 in 2009; 95 in 2010; 163 in 2011; and 239 in 2012. This showed that LSG has become an important bariatric procedure and represents 50% of the bariatric workload in our unit currently. Nevertheless, there has been a declining trend in the number of patients returning for clinic follow-up over the years. In 5 years, only nine (7.6%) out of a total 119 patients were able to return to clinic for long-term follow-up. Through telephone interviews, the follow-up data of 61 patients (51.2%) were available at 5 years.
3.2. Outcome of revisional surgery
With regard to revisional procedure after primary LSG, a total 18 patients (2.3%) needed surgical revision due to various reasons: weight regain (n = 6), persistent type 2 diabetes (n = 2), stricture (n = 2), and GERD (n = 8). All revisions were performed laparoscopically. The type of revisional procedure performed were RYGB (n = 16) and DS (n = 2) with a median time to revision of 33 months (range 3–62 months). The earliest intervention (3 months) was for a patient who had persistent vomiting due to the gastric tube stricture. The mean surgical time for revisional surgery was 152.2 minutes (range 95–205 minutes) with average blood loss of 30 mL (range 20–70 mL). The postoperative stay was short at 3.7 days (range 2–7 days). There was only one minor complication due to wound infection and no major complications occurred in our revision series. All symptoms (stricture and GERD) due to complicated LSG resolved immediately after surgery. For patients with weight regain (prerevision BMI 34.9 kg/m2), the revisional surgery resulted in mean excess weight loss of 54.0% with mean BMI 24.9 kg/m2 at 6 months postoperatively. For patients with poor glycemic control (n = 2), there was a 23.7% mean reduction of HbA1c with one patient in complete remission without medication in 1 year.
4. Discussion
This study has confirmed the long-term efficacy of LSG as a stand-alone bariatric procedure. Our long-term results over 5 years has shown that primary LSG resulting in > 70% average % EWL. This result concurs with recent publications which showed > 50% EWL in long-term results,12; 13; 14; 15; 16; 17; 18; 19 ; 20 as shown in Table 3.12; 13; 14; 15; 16; 17; 18; 19; 20 ; 21 Nine of these reports were from Western countries and two were from Asia including the present study. However, our study reported greater weight loss which might be due to our cohort of patients having a lower BMI (37.3 kg/m2), thus resulting in greater efficacy of weight loss as compared with other series. Besides, we used a smaller bougie size, 36 F for calibration to make a tighter sleeve which may have facilitated greater weight loss. However, if we correlate the % EWL with different BMI groups, the lower BMI patients (< 35 kg/m2) seemed to derive greater weight loss as compared with higher BMI patients (Fig. 1B). However, the use of % EWL might be exaggerated in lower BMI patient as it depends on the patients initial BMI as a relative measure. The heavier the patient, the smaller the % EWL, and vice versa. Further studies are required to find a more suitable parameter for weight loss results for the outcome of bariatric surgery.
| Study | n | Bougie size, F | Follow-up, y | Initial BMI | BMI at follow-up | % EWL | %GERD |
|---|---|---|---|---|---|---|---|
| Rawlins et al16 (2013) | 49 | 24 | 5 | 65.0 | NA | 86.0 | 11.0 |
| Weiner et al19 (2007) | 8 | 32, 44 | 5 | 60.7 | 45.0 | NA | NA |
| Strain et al18 (2011) | 23 | 40 | 5 | 52.2 | 40.0 | NA | NA |
| Catheline et al13 (2013) | 45 | 34 | 5 | 49.1 | 35.4 | 50.7 | 33.3 |
| Bohdjalian et al12 (2010) | 21 | 48 | 5 | 48.2 | NA | 55.0 | 30.8 |
| Sarela et al17 (2012) | 13 | 32 | 8+ | 45.8 | NA | 68.0 | NA |
| D'Hondt et al14 (2011) | 23 | 30 | 6 | 39.3 | NA | 55.9 | NA |
| Himpens et al15 (2010) | 30 | 34 | 6+ | 39.0 | 31.1 | 53.3 | 23.0 |
| Braghetto et al21 (2012) | 60 | NA | 5 | 38.4 | 29.9 | 57.3 | 27.5 |
| Zachariah et al20 (2013) | 6 | 36 | 5 | 37.4 | 27.9 | 63.7 | NA |
| Present study | 61 | 36 | 5 | 37.3 | 26.2 | 72.6 | 17.0 |
Data are presented as mean.
% EWL = percentage excess weight loss; BMI = body mass index; GERD = gastro-esophageal reflux disease; NA = not available.
Our results showed the maximal weight loss occur within 2 years after surgery with tendency for weight regain later in our series. Similarly, Braghetto et al21 reported weight regain was observed in 30% of patients with the trend starting after the third year of surgery. The reasons for weight regain could be due to factors such as gastric tube dilatation or changes in eating habit by shifting toward “easy” highly caloric food.22 It has been suggested different gastric tube sizes would affect the effect of weight loss. The smaller the sleeve gastric lumen, the less likely for gastric tube dilatation19 based on Laplaces law but it might be associated with higher complication rates such as GERD, leaks, and strictures.23 The recent consensus regarding the optimal bougie size for LSG were 32–36 F for final calibration.23 The second consideration is the complete removal of gastric fundus, the site of production of hormone ghrelin as it will increase postprandial sensation of satiety after meals, thus maintaining the effect of long-term sustainable weight loss. Furthermore, the role of ghrelin effect has been strongly supported by an animal experimental study which showed that replacement of ghrelin in gastrectomy mice would lead to weight regain.24 Despite all these technical considerations, the most important modality for durable weight loss still relies on patients' compliance with good eating habits as weight recidivism would occur in any bariatric surgery.25 Avoiding easy calorie dense food with a good lifestyle is the corner stone for long-term weight loss. Therefore, continued patient education within multidisciplinary teams is important for the success of any bariatric program.
With effective weight loss, most of the obesity-related comorbidities were significantly reduced in our series especially diabetes mellitus. Although any effective weight loss would lead to better glycemic effects due to reduced systemic inflammatory response and improvement of insulin sensitivity, LSG might have metabolic reduction effect as studies have shown it has hormonal effects with raised peptides YY and GLP-1.26 ; 27 Melissas et al28 further explained the incretins effect might be due to early and prolonged stimulation of intestinal L cells due to the extension of food contact time with the area of the terminal ileum occurs after LSG with short bowel transit, thus enhancing the glycemic control.
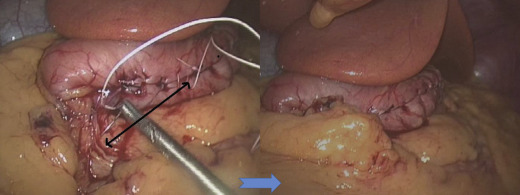
The surgical risk of LSG has decreased dramatically after the learning curve. Our series showed high incidence of leaks in the first 100 cases (9%) but low leakage rates in subsequent series due to improved surgical techniques. The formation of long and narrow gastric tubes will cause stricture if the gastric tube is sleeved too narrow at the incisura angularis angle. Usually endoscopic dilatation is effective at relieving the stricture but in difficult cases with long stricture, revision with roux-en Y gastric bypass is needed.29 Besides, laparoscopic seromyotomy of stricture areas has been used with good outcomes.30 Furthermore, we recognized that the slim gastric tube tend to coil medially once formed which would aggravate the severity of strictures, therefore it is essential to fix the gastric tube to the retroperitoneal tissue as an L-configuration tube (Fig. 2).
|
|
|
Figure 2. Fixation of gastric tube to retroperitoneum. |
The main long-term drawback of LSG is the development of GERD. The available published results on GERD in long-term results of LSG showed 11–33% reflux rate (Table 3). Our experience with LSG showed de novo GERD occurred up to 17% in our long-term series. Although GERD is a complex disease associated with obesity with proposed pathophysiology due to an increased prevalence of hiatus hernia, increased intraabdominal pressure, delayed gastric emptying, and dysfunctioning of the lower esophageal sphincter. However, the relationship between sleeve gastrectomy and GERD is intriguing as various studies reported that it either improves or worsens reflux symptoms after surgery. Chiu et al 31 reported a systemic review on sleeve gastrectomy on GERD which did not show any conclusive relationships on both of them. As the stapling of fundus near the angle of His which would usually disrupt the cardio-esophageal junction, it is logical to expect the prevalence of GERD will be higher in combination with high intragastric pressure after surgery. Besides, alterations in gastric emptying after LSG could be potentially important in determining its effect on GERD. However, the study of sleeve gastrectomy on gastric emptying is conflicting 28; 32 ; 33 which might be due to different surgical techniques used. Recently, there was a suggestion that a combination of dilated upper part of the sleeve with a relative narrowing of the midstomach, without complete obstruction, might contribute to GERD after sleeve gastrectomy.34 This functional obstruction would result in severe esophageal dysmotility with reflux symptom. However, patients who had sleeve gastrectomy with concomitant repair of hiatal hernia (HH) were found to have reduced incidence of GERD after surgery.35 The argument is that the presence of HH could further impair the antireflux mechanisms, worsening the severity of GERD. Therefore, currently it is recommended and essential to look for hiatal defects and repair it during sleeve gastrectomy.23 Based on our experience, small hiatal defects could be missed at preoperative endoscopy or upper gastrointestinal contrast study. However, with careful examination of the crura and proper dissection of the left crura during dissection of the angle of His, the small crural defect would be revealed easily.
Long-term revisional rates of LSG is lower than the previous experience of laparoscopic gastric banding and laparoscopic vertical banded gastroplasty, and similar to gastric bypass. Our result demonstrated the revision rate for LSG was low (2.2%). Most of the revisions were for intractable GERD or weight regain. As LSG is seen as a restrictive procedure, some weight regain is also expected in longer term follow-ups. In patient with weight regain without reflux symptoms, duodenal-jejunal bypass36 or duodenal switch is a good option. Although revisional procedures for weight regain with another restrictive procedures such as adding gastric banding,37 resleeve gastrectomy38 have been described, we believe malabsorptive procedures should be employed instead to prevent further failure. For GERD, the definitive treatment would be ideally RYGB. Although LSG is a good procedure for bariatric surgery, the efficacy of diabetes remission in lower BMI patient is inferior to the gastric bypass.39 Similarly, in the Schauer et al study,40 although the diabetes remission rate was similar between gastric bypass ad sleeve gastrectomy, 30% of the sleeve patients were on glycemic agents but none in gastric bypass patients. Therefore, in patients with persistent diabetes after LSG, adding a duodenal switch might be a good option based on the mechanism of foregut bypass. Our revision series showed that low complications could be achieved in experienced hands. During revision to RYGB, it is important to ensure good vascularity of proximal gastric tube and refashion the gastric pouch with larger stapler height due to gastric tube scarring.
The limitation of this study is its retrospective nature of analysis and a low clinic follow-up rate (7.6%) in 5 years outcome data. There was a declining number of patients returning for clinic follow-ups as most of the patients achieved the desired weight loss with minimal side effects or nutrition issues unless complications occurs. In other words, they did well with the operation, however, our practice is diligent with calling patients regularly for follow-up and auditing the outcome. Although only nine out of our 119 patients from the 5-years outcome series have regular clinic follow-ups, we were able to trace 52 patients by telephone interview, making the total number of 61 (51.2%) patients for 5 years outcome data. In fact, this is the largest number of follow-up patients for 5 years outcome data reported so far (Table 3). As most of the bariatric surgery series15 ; 19 also reported the difficulty of attaining long-term follow-up, thus it is essential to reinforce patient commitment of follow-up at least on annual basis.
5. Conclusion
We report the long-term outcome of primary LSG and its revision rate. The primary LSG is a safe and durable primary bariatric procedure with overall 70% EWL at 5 years and satisfactory resolution of comorbidities. The need of revision of LSG is low (2.2%) and indicated mainly for refractory GERD or weight regain with satisfactory outcome. However, GERD is still a major problem associated with LSG, which is up to 17% symptoms in 5 years and required further investigation.
Acknowledgments
This study was supported by University of Malaya Research Grant No. RG433/12HTM (Kuala Lumpur, Malaysia).
References
- 1 D.S. Hess, D.W. Hess; Biliopancreatic diversion with a duodenal switch; Obes Surg, 8 (1998), pp. 267–282
- 2 J.P. Regan, W.B. Inabnet, M. Gagner, A. Pomp; Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the supersuper obese patient; Obes Surg, 13 (2003), pp. 861–864
- 3 D. Cottam, F.G. Qureshi, S.G. Mattar, et al.; Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity; Surg Endosc, 20 (2006), pp. 859–863
- 4 D. Nocca, D. Krawczykowsky, B. Bomans, et al.; A prospective multicenter study of 163 sleeve gastrectomies: results at 1 and 2 years; Obes Surg, 18 (2008), pp. 560–565
- 5 M. Deitel, R.D. Crosby, M. Gagner; The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25–27, 2007; Obes Surg, 18 (2008), pp. 487–496
- 6 M. Gagner, M. Deitel, T.L. Kalberer, A.L. Erickson, R.D. Crosby; The Second International Consensus Summit for Sleeve Gastrectomy, March 19–21, 2009; Surg Obes Relat Dis, 5 (2009), pp. 476–485
- 7 M. Deitel, M. Gagner, A.L. Erickson, R.D. Crosby; Third International Summit: current status of sleeve gastrectomy; Surg Obes Relat Dis, 7 (2011), pp. 749–759
- 8 M.M. Hutter, B.D. Schirmer, D.B. Jones, et al.; First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass; Ann Surg, 254 (2011), pp. 410–420 discussion 20–22
- 9 K.H. Ser, W.J. Lee, Y.C. Lee, J.C. Chen, Y.H. Su, S.C. Chen; Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: staple-line reinforcement is important for preventing leakage; Surg Endosc, 24 (2010), pp. 2253–2259
- 10 W.J. Lee, J.C. Chen, W.C. Yao, J.J. Taou, Y.C. Lee, K.H. Ser; Transumbilical 2-site laparoscopic Roux-en-Y gastric bypass: initial results of 100 cases and comparison with traditional laparoscopic technique; Surg Obes Relat Dis, 8 (2012), pp. 208–213
- 11 C.C. Chiu, W.J. Lee, W. Wang, P.L. Wei, M.T. Huang; Prevention of trocar-wound hernia in laparoscopic bariatric operations; Obes Surg, 16 (2006), pp. 913–918
- 12 A. Bohdjalian, F.B. Langer, S. Shakeri-Leidenmuhler, et al.; Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin; Obes Surg, 20 (2010), pp. 535–540
- 13 J.M. Catheline, M. Fysekidis, I. Bachner, et al.; Five-year results of sleeve gastrectomy; J Visc Surg, 20 (2013), pp. 00104–00105
- 14 M. D'Hondt, S. Vanneste, H. Pottel, D. Devriendt, F. Van Rooy, F. Vansteenkiste; Laparoscopic sleeve gastrectomy as a single-stage procedure for the treatment of morbid obesity and the resulting quality of life, resolution of comorbidities, food tolerance, and 6-year weight loss; Surg Endosc, 25 (2011), pp. 2498–2504
- 15 J. Himpens, J. Dobbeleir, G. Peeters; Long-term results of laparoscopic sleeve gastrectomy for obesity; Ann Surg, 252 (2010), pp. 319–324
- 16 L. Rawlins, M.P. Rawlins, C.C. Brown, D.L. Schumacher; Sleeve gastrectomy: 5-year outcomes of a single institution; Surg Obes Relat Dis, 9 (2013), pp. 21–25
- 17 A.I. Sarela, S.P. Dexter, M. O'Kane, A. Menon, M.J. McMahon; Long-term follow-up after laparoscopic sleeve gastrectomy: 8–9-year results; Surg Obes Relat Dis, 8 (2012), pp. 679–684
- 18 G.W. Strain, T. Saif, M. Gagner, M. Rossidis, G. Dakin, A. Pomp; Cross-sectional review of effects of laparoscopic sleeve gastrectomy at 1, 3, and 5 years; Surg Obes Relat Dis, 7 (2011), pp. 714–719
- 19 R.A. Weiner, S. Weiner, I. Pomhoff, C. Jacobi, W. Makarewicz, G. Weigand; Laparoscopic sleeve gastrectomy: influence of sleeve size and resected gastric volume; Obes Surg, 17 (2007), pp. 1297–1305
- 20 S.K. Zachariah, P.C. Chang, A.S. Ooi, M.C. Hsin, J.Y. Kin Wat, C.K. Huang; Laparoscopic sleeve gastrectomy for morbid obesity: 5 years experience from an Asian center of excellence; Obes Surg, 23 (2013), pp. 939–946
- 21 I. Braghetto, A. Csendes, E. Lanzarini, K. Papapietro, C. Carcamo, J.C. Molina; Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results; Surg Laparosc Endosc Percutan Tech, 22 (2012), pp. 479–486
- 22 J. Himpens, G. Dapri, G.B. Cadiere; A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years; Obes Surg, 16 (2006), pp. 1450–1456
- 23 R.J. Rosenthal, A.A. Diaz, D. Arvidsson, et al.; International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of > 12,000 cases; Surg Obes Relat Dis, 8 (2012), pp. 8–19
- 24 C. Dornonville de la Cour, A. Lindqvist, E. Egecioglu, et al.; Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice; Gut, 54 (2005), pp. 907–913
- 25 S. Karmali, B. Brar, X. Shi, A.M. Sharma, C. de Gara, D.W. Birch; Weight recidivism postbariatric surgery: a systematic review; Obes Surg, 23 (2013), pp. 1922–1933
- 26 M. Tsoli, A. Chronaiou, I. Kehagias, F. Kalfarentzos, T.K. Alexandrides; Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study; Surg Obes Relat Dis, 9 (2013), pp. 667–677
- 27 D. Papamargaritis, C.W. le Roux, E. Sioka, G. Koukoulis, G. Tzovaras, D. Zacharoulis; Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy; Surg Obes Relat Dis, 9 (2013), pp. 192–201
- 28 J. Melissas, A. Leventi, I. Klinaki, et al.; Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study; Ann Surg, 258 (2013), pp. 976–982
- 29 A. Lacy, A. Ibarzabal, E. Pando, et al.; Revisional surgery after sleeve gastrectomy; Surg Laparosc Endosc Percutan Techn, 20 (2010), pp. 351–356
- 30 G. Dapri, G.B. Cadiere, J. Himpens; Laparoscopic seromyotomy for long stenosis after sleeve gastrectomy with or without duodenal switch; Obes Surg, 19 (2009), pp. 495–499
- 31 S. Chiu, D.W. Birch, X. Shi, A.M. Sharma, S. Karmali; Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review; Surg Obes Relat Dis, 7 (2011), pp. 510–515
- 32 S. Shah, P. Shah, J. Todkar, M. Gagner, S. Sonar, S. Solav; Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus; Surg Obes Relat Dis, 6 (2010), pp. 152–157
- 33 H. Bernstine, R. Tzioni-Yehoshua, D. Groshar, et al.; Gastric emptying is not affected by sleeve gastrectomy: scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum; Obes Surg, 19 (2009), pp. 293–298
- 34 A. Keidar, L. Appelbaum, C. Schweiger, R. Elazary, A. Baltasar; Dilated upper sleeve can be associated with severe postoperative gastroesophageal dysmotility and reflux; Obes Surg, 20 (2010), pp. 140–147
- 35 E. Soricelli, A. Iossa, G. Casella, F. Abbatini, B. Cali, N. Basso; Sleeve gastrectomy and crural repair in obese patients with gastroesophageal reflux disease and/or hiatal hernia; Surg Obes Relat Dis, 9 (2013), pp. 356–361
- 36 W.J. Lee, K.T. Lee, K. Kasama, et al.; Laparoscopic single-anastomosis duodenal–jejunal bypass with sleeve gastrectomy (SADJB–SG): short-term result and comparison with gastric bypass; Obes Surg, 24 (2014), pp. 109–113
- 37 A.J. Greenstein, B.P. Jacob; Placement of a laparoscopic adjustable gastric band after failed sleeve gastrectomy; Surg Obes Relat Dis, 4 (2008), pp. 556–558
- 38 A. Baltasar, C. Serra, N. Perez, R. Bou, M. Bengochea; Resleeve gastrectomy; Obes Surg, 16 (2006), pp. 1535–1538
- 39 W.J. Lee, K. Chong, K.H. Ser, et al.; Gastric bypass versus sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial; Arch Surg, 146 (2011), pp. 143–148
- 40 P.R. Schauer, S.R. Kashyap, K. Wolski, et al.; Bariatric surgery versus intensive medical therapy in obese patients with diabetes; N Engl J Med, 366 (2012), pp. 1567–1576
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?