(Created page with "==Summary== Cerebellar mutism is a transient period of speechlessness that evolves after posterior fossa surgery in children. Although direct cerebellar and brain stem injury...") |
m (Scipediacontent moved page Draft Content 787616378 to Kara-Gedik et al 2014a) |
(No difference)
| |
Latest revision as of 11:31, 26 May 2017
Summary
Cerebellar mutism is a transient period of speechlessness that evolves after posterior fossa surgery in children. Although direct cerebellar and brain stem injury and supratentorial dysfunction have been implicated in the mediation of mutism, the pathophysiological mechanisms involved in the evolution of this kind of mutism remain unclear. Magnetic resonance imaging revealed dentatothalamocortical tract injuries and single photon emission computed tomography showed cerebellar and cerebral hypoperfusion in patients with cerebellar mutism. However, findings with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in this group of patients have not been documented previously. In this clinical case, we report a patient who experienced cerebellar mutism after undergoing a posterior fossa surgery. Right cerebellar and left frontal lobe hypometabolism was shown using FDG PET/CT. The FDG metabolism of both the cerebellum and the frontal lobe returned to normal levels after the resolution of the mutism symptoms.
Keywords
cerebellum;FDG;mutism;PET/CT
1. Introduction
Posterior fossa syndrome consists of transient cerebellar mutism, cognitive symptoms, and neurobehavioral abnormalities.1 It was first reported in 1985 as a complication of posterior fossa surgery.2 Although a transient nature was recognized as the hallmark of this type of mutism, development after the resection of a cerebellar mass lesion, delayed onset after intervention, and association with other neurological, emotional, and behavioral disturbances were described as core features of this syndrome.3
The incidence of postoperative cerebellar mutism ranges between 11% and 29%.4 It is more commonly observed after the resection of posterior fossa tumors in children; however, trauma, vascular incidents, or infections may also be followed by this complication.4 Patients with medulloblastomas and/or brain stem invasion are at a greater risk of experiencing this condition compared with those who have pilocytic astrocytoma or ependymoma.5
After the first description of this syndrome appeared, more than 400 cases with cerebellar mutism have been reported, and several hypotheses have been suggested to explain the clinical features of this complex syndrome. Because surgery is the primary event, surgical injury to dentate nuclei of the cerebellum has been proposed as the primary cause of the initiation of mutism. However, the delayed onset of mutism after surgery and the presence of concomitant neurological symptoms have raised questions about the association of supratentorial structures to this clinical picture. On one hand, there were some studies with single photon emission computed tomography (SPECT) showing cerebellar hypoperfusion in mute patients with normalization of blood flow after mutism had resolved6 ; 7; on the other hand, SPECT studies linking mutism to hypoperfusion of contralateral frontal cortex or other supratentorial targets were also reported.1 ; 8 This asymmetric blood flow in cortical areas contralateral to a remote cerebellar lesion, which is described previously as crossed cerebello-cerebral diaschisis, is a well-known phenomenon, and injury to the dentatothalamocortical pathway is suggested as the cause.3
We describe a patient who underwent cerebellar surgery and developed mutism after the procedure. Metabolic imaging with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) was performed at both mutistic and postmutistic phases. The FDG metabolism of the primary surgical site and the cortical areas is discussed, and the literature is reviewed.
2. Case report
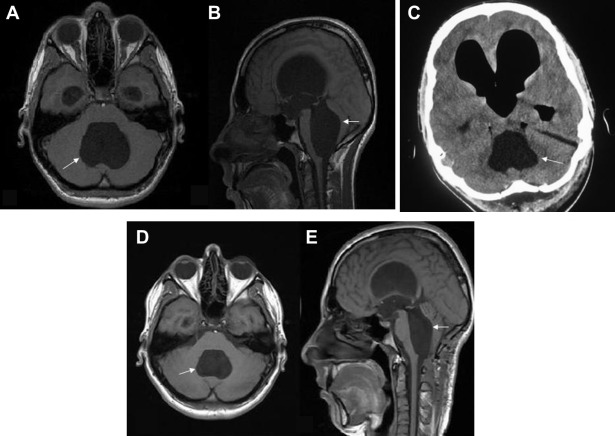
A 17-year-old male patient was admitted to the hospital complaining of a headache. His history showed that he has been suffering from headaches and vomiting for 4 months and 3 weeks, respectively. His Glascow Coma Scale was 15, and his neurological examination yielded completely normal results. Cranial magnetic resonance imaging (MRI) showed a large arachnoid cyst formation (47 × 36 × 61 mm) at the fourth ventricle with significant brain stem and cerebellum compression accompanied with mild tetraventricular hydrocephalus (Fig. 1A and B). The patient underwent an operation at the neurosurgery department. The operation was performed with the patient placed in a sitting position, and a midline suboccipital approach was used. After the suboccipital craniotomy, the dura was opened and both cerebellar hemispheres were retracted laterally. After the splitting of the vermis, under microscopic visualization, the thickened membrane of the large arachnoid cyst, which was located at the floor of the fourth ventricle, was perforated (cystoventriculostomy). The anterior–inferior part of the arachnoid cyst was also fenestrated into the cerebellomedullary cisterns (cystocisternostomy).
|
|
|
Figure 1. Preoperative (A) axial and (B) sagittal T1-weighted magnetic resonance imaging show large arachnoid cyst formation at the fourth ventricle (arrow) with significant brain stem and cerebellum compression. Pneumocephalus and ventricular dilatation are shown on (C) postoperative computed tomography. T1-weighted (D) axial and (E) sagittal magnetic resonance imaging performed 3 months after surgery reveal a decrease in cyst size (arrow) as well as compression of the cerebellum and brain stem. |
The early postoperative neurological examination was uneventful until the 2nd postoperative day. Twenty-four hours after the surgery, results of his neurological examination showed that his condition had deteriorated. Cerebellar mutism-like symptoms such as speechlessness, cerebellar ataxia, and apathia developed.
The patient was imaged anatomically and metabolically at the mutistic phase. His CT scan (Fig. 1C) revealed dilatation and pneumocephalus in all ventricles including right and left lateral ventricles and the fourth ventricle. However, no structural abnormality was noted.
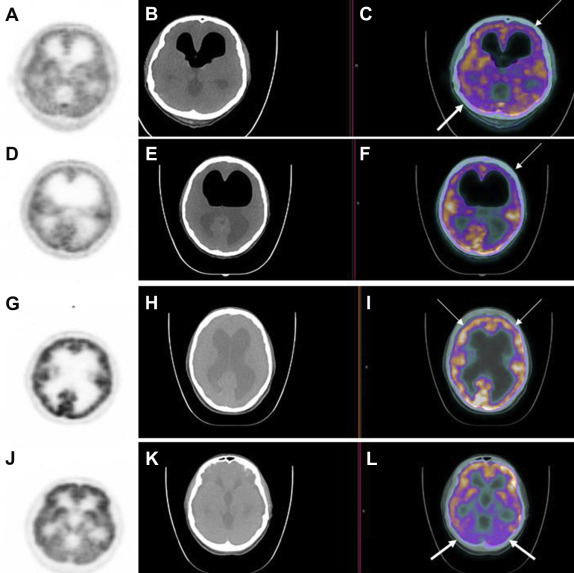
Metabolic imaging with dual modality PET/CT examination was obtained 60 minutes after the intravenous administration of 7 mCi FDG, using an integrated scanner (Biograph mCT, Siemens, Germany; Fig. 2A–L). The patient was kept at rest in a silent room after the injection of a radiopharmaceutical. CT was performed without intravenous contrast injection, with 120 kV, 35 mA, a pitch of 1.5, a section thickness of 5 mm, and a field of view of 70 cm. The PET scan was started immediately after the unenhanced CT. Standardized uptake value (SUV) was calculated using the “region of interest” technique. Relative hypometabolism was shown both in the right cerebellar hemisphere (Fig. 2A and C) and the left frontal lobe (Fig. 2A, C, D and F) with PET/CT at the mutistic phase. Whereas the ratio of SUV mean values of right to left cerebellar hemisphere was calculated as 0.82, the ratio of SUV mean values of left to right frontal lobe was calculated as 0.80 at the mutistic phase. Ventricular dilatation and pneumocephalus findings were also noted. Physologic FDG biodistribution was seen in the remaining parts of the body.
|
|
|
Figure 2. (A, D) PET, (B, E) CT, and fused (C, F) PET/CT images obtained at the mutistic phase. Relative FDG hypometabolism in the right cerebellar hemisphere [(C) thick arrow, fused PET/CT image] and left frontal lobe [(C, F) thin arrows, fused PET/CT image] is shown. (G, J) PET, (H, K) CT, and (I, L) fused PET/CT imaging are repeated at the postmutistic phase. Fused PET/CT images showed symmetric FDG metabolism in the frontal lobes [(I) thin arrows, fused PET/CT image] and in the cerebellar hemispheres [(L) thick arrows, fused PET/CT image]. CT = computed tomography; FDG = 18F-fluorodeoxyglucose; PET = positron emission tomography. |
Regression in mutism symptoms was observed 3 months after the surgery. The patient recovered spontaneously; headache and vomiting symptoms were controlled. PET/CT examination was repeated at the postmutistic phase under the same physical conditions, and it revealed that FDG metabolism of the frontal lobes (Fig. 2G and I) and the cerebellum (Fig. 2J and L) were symmetric; no relative hypometabolism was observed. At the postmutistic phase, the ratio of SUV mean values of right to left cerebellar hemisphere was calculated as 1.03, and the ratio of SUV mean values of left to right frontal lobes was calculated as 1.01. The cranial MRI, which was performed 3 months after the surgery, showed a marked decrease in cyst size (37 × 29 × 54 mm) as well as compression findings in the cerebellum and the brain stem (Fig. 1D and E).
3. Discussion
Posterior fossa syndrome and cerebellar mutism may occur after the resection of cerebellar tumors. The pathophysiology and anatomic basis of this syndrome have not been well understood yet, but a variety of mechanisms have been suggested in previous studies. In some studies, the development of mutism was attributed to the transient dysfunction of the dopaminergic cell group in the mesencephelon.9 Postoperative vasospasm of cerebellar or brain stem territories, surgical manipulation of cerebellum, intraoperative coagulation of perforating vessels, and arterial embolic occlusion were also discussed in the evolving process of cerebellar mutism.10 Taking into consideration the association between the cerebellum and cognitive and linguistic functions, which depend on the functional connection of the lateral part of the right cerebellum to the left prefrontal area, the authors wondered whether cortical areas play a role in the generation of this syndrome by trans-synaptic reflex mechanisms. This hypothesis carried the discussion to an area in which diaschisis was reported as a conceivable explanation behind this syndrome. In patients with cerebellar mutism, functional depression of cerebello–thalamo–cerebral pathways and deprivation of cerebellar input have been implicated to induce cerebral circulatory and metabolic hypofunction.8 Hypoperfusion of the cerebral hemispheres with SPECT radiotracers has been shown; however, the FDG metabolism of the cerebellum and the cerebrum during the course of this clinical picture has not been previously demonstrated with PET/CT.
Neuroimaging of the central nervous system by scintigraphy can be performed with SPECT or PET. SPECT was first introduced in the 1960s to detect breakdowns in the blood–brain barrier, and PET with FDG was later developed to measure regional cerebral glucose metabolism.11 Blood flow and metabolism are coupled in most pathological entities, and the changes seen on SPECT brain perfusion associate with the corresponding abnormalities on FDG PET.11
The hallmark features of diaschisis are hypoperfusion, decreased oxygen consumption, and hypometabolism, which can be shown by MRI or with SPECT studies. In crossed cerebello-cerebral diaschisis, it is suggested that damage to the efferent cerebellar pathway, which is often invaded by posterior fossa tumors and prone to surgical damage during resection, deprives the cerebral cortex of cerebellar input. Significant decreases in cerebral blood flow within the frontal regions have been reported with contrast-enhanced perfusion MRI in patients with posterior fossa syndrome by Miller et al.8 Catsman-Berrevoets and Aarsen3 reported hypoperfusion of the frontal cortical regions in most of the children they have studied with Tc-99m hexamethylpropyleneamine SPECT in posterior fossa syndrome. A significant improvement of frontal perfusion deficits in parallel to the clinical remission of mutism was shown with SPECT by De Semet et al.1 In an adult patient who experienced cognitive, linguistic, and affective disturbances after a right superior cerebellar artery infarction, relative hypoperfusion in the right cerebellar hemisphere and left medial frontal lobe was depicted with Tc-99m ethylcysteinate dimer SPECT study by Marien et al.12
In our case, relative FDG hypometabolism was shown in the right cerebellar hemisphere and the left frontal lobe, and the presence of hypometabolism—which, although always suggested, was not previously demonstrated in posterior fossa syndrome—was proven. No relative FDG hypometabolism was noted after the recovery of the symptoms. The SUV mean ratio of right to left cerebellar hemisphere was 0.82 during the mutistic phase, which then changed to 1.03 at the recovery stage. Similarly, the left to right SUV mean ratio of frontal lobes was 0.80 and 1.01 at the mutistic and postmutistic phases, respectively. Because, in our case, the operation was performed through the cerebellar vermian lobe, we thought that FDG hypometabolism was generated secondary to cerebellar manipulation during surgery. Vasospasm and edema might have occurred during surgery, which induced hypoperfusion in the cerebellum or its outflow tract, which deprived the cerebral cortex from cerebellar input. This disconnection between the cerebellar projections to thalami and cerebral cortex resulted in metabolic hypofunction in the cerebrum. Hypometabolism in the left frontal lobe may also evolve secondary to the compressive effect of pneumocephalus and dilated ventricle, which was more prominent in the left lateral ventricle. However, there was also dilatation in the fourth ventricle and although it was a uniform dilatation, there was still relative hypometabolism in the right cerebellar hemisphere. Considering the presence of right cerebellar and left frontal hypometabolism, we thought that hypometabolism, which resolved at the postmutistic phase, was generated secondary to crossed cerebro-cerebellar diaschisis instead of the compressive effect of dilatation.
The question why mutism manifests after a latency period remains unanswered in the literature. The most probable explanation is the coexisting cerebellar and cortical dysfunction. Because cerebellar deficits should be apparent directly after cerebellar injury, they cannot explain the delayed onset of mutism. Postmutistic symptoms also show variability. Because vasospasm or edema are the suggested primary effects of surgery (which initiates hypoperfusion and hypometabolism), normalization of blood flow provides perfusion, thereby allowing metabolism of the affected region to return to the normal level. The fact that hypometabolism was observed both in the cerebellum and the cerebrum and eventually resolved after the recovery period, also supports the idea that cerebellar injury alone cannot fully explain the cognitive and emotional deficits observed in posterior fossa syndrome. Our case also shows that unilateral cerebellar and cerebral hypometabolism may be observed in this syndrome instead of bilateral involvement. Because full recovery without any sequela was observed in our patient, we thought that unilateral involvement may be associated with reduced postoperative morbidity.
We report the reduced FDG metabolism in the cerebellum and the cerebrum with PET/CT in a patient with cerebellar mutism for the first time in the literature. The metabolic depression of frontal lobe strengthens the hypothesis that mutism in posterior fossa syndrome is a speech apraxia, rather than a simple dysarthria.
References
- 1 H.J. De Semet, H. Baillieux, P. Wackenier, et al.; Long-term cognitive deficits following posterior fossa tumor resection: a neuropsycological and functional neuroimaging follow-up study; Neuropsychology, 23 (2009), pp. 694–704
- 2 H.L. Rekate, R.L. Grubb, D.M. Aram, J.F. Hahn, R.A. Ratcheson; Muteness of cerebellar origin; Arch Neurol, 42 (1985), pp. 697–698
- 3 C.E. Catsman-Berrevoets, F.K. Aarsen; The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery; Cortex, 46 (2010), pp. 933–946
- 4 T. Gudrunardottir, A. Sehested, M. Juhler, K. Schmiegelow; Cerebellar mutism; Child Nerv Syst, 27 (2011), pp. 355–363
- 5 M. Küper, D. Timmann; Cerebellar mutism; Brain Lang, 127 (2013), pp. 327–333
- 6 M. Nishikawa, M. Komiyana, H. Sakamoto, T. Yasui, H. Nakajima; Cerebellar mutism after basilar artery occlusion—case report; Neurol Med Chir, 38 (1998), pp. 569–573
- 7 Y. Ersahin; SPECT in cerebellar mutism; Child Nerv Syst, 14 (1998), pp. 611–613
- 8 N.G. Miller, W.E. Reddick, M. Kocak, et al.; Cerebello-cerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors; Am J Neuroradiol, 31 (2010), pp. 288–294
- 9 C.E. Catsman-Berrevoets, H.R. Van Dongen, C.P. Zwetsloot; Transient loss of speech followed by dysarthria after removal of a posterior fossa tumour; Dev Med Child Neurol, 34 (1992), pp. 1102–1117
- 10 L. Ferrante, L. Mastronardi, M. Acqui, A. Fortuna; Mutism after posterior fossa surgery in children. Report of three cases; J Neurosurg, 72 (1990), pp. 959–963
- 11 A. Alavi, L.J. Hirsch; Studies of central nervous system disorders with single photon emission computed tomography and positron emission tomography: evolution over the past 2 decades; Semin Nucl Med, 21 (1991), pp. 58–81
- 12 P. Marien, H. Baillieeux, H.Y. De Semet, et al.; Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study; Cortex, 45 (2009), pp. 527–536
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?