(Created page with "==Summary== ====Background==== Although nonoperative management for perforated peptic ulcer (PPU) has been used for several decades, the indication is still unclear. A clini...") |
m (Scipediacontent moved page Draft Content 265573351 to Cao et al 2014a) |
(No difference)
| |
Latest revision as of 11:30, 26 May 2017
Summary
Background
Although nonoperative management for perforated peptic ulcer (PPU) has been used for several decades, the indication is still unclear. A clinicoradiological score was sought to predict who can benefit from it.
Methods
A clinicoradiological protocol for the assessment of patients presenting with PPU was used. A logistic regression model was applied to identify determinant variables and construct a clinical score that would identify patients who can be successfully treated with nonoperative management.
Results
Of 241 consecutive patients with PPU, 107 successfully received nonoperative management, and 134 required surgery. In multivariable analysis, the following four variables correlated with surgery and were given one point each toward the clinical score: age ≥70 years, fluid collection detection by ultrasound, contrast extravasation detection by water-soluble contrast examination, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥8. Eighty-five percent of patients with a score of 1 or less were successfully treated with nonoperative management, whereas 23 of 29 patients with a score of 3 or more required surgery. The area under the receiver operating characteristic curve was 0.804 (95% confidence interval = 0.717–0.891).
Conclusion
By combining clinical, radiological parameters, and APACHE II score, the clinical score allowed early identification of PPU patients who can benefit from nonoperative management.
Keywords
perforated peptic ulcer;surgery;treatment
1. Introduction
Because of the widespread clinical use of H2 receptor antagonists and proton-pump inhibitors, significantly less elective surgery has been carried out for uncomplicated peptic ulcer. However, the complications associated with peptic ulcer, especially perforation, are still common in recent years.1 ; 2 In 1946, Taylor first reported a series of 28 patients with perforated peptic ulcer (PPU) receiving nonoperative management. The mortality rate was 14%, which was less than the approximate 20% following a direct simple closure with omental patch.3 With the development of critical care medicine, the mortality of PPU treated by the nonoperative approach has significantly reduced.4; 5 ; 6 However, the indication of nonoperative management is unclear, and urgent repair of perforation is still the standard approach for PPU in many clinical centers. The aim of this study is to establish a clinicoradiological scoring system to predict who can benefit from it.
2. Materials and methods
2.1. Patients
Between January 2002 and December 2010, all patients who presented at the general surgery unit of our hospital with clinical symptoms of PPU confirmed by abdominal plain film with pneumoperitoneum were evaluated for inclusion in this study. Patients with PPU history or suspected with gastric cancer were excluded from this study. Patients with severe diffuse peritonitis or septic shock received direct surgery after fluid resuscitation. Otherwise, the patients were initially managed nonoperatively. The study was conducted in accordance with the institutional guidelines of the Xuanwu Hospital Ethics Committee, Beijing, China.
Demographic data were collected for gender and age (continuous data, divided into the following two categories: <70 years and ≥70 years). Clinical variables included duration of abdominal pain prior to admission, the presence of fever with a threshold of 38°C, use of steroids or nonsteroidal anti-inflammatory drugs, the presence of Helicobacter pylori infection, and satiety during perforation. Laboratory variables were leukocyte count with a threshold of 12 × 109/L and albumin level with a threshold of 30 g/L. Radiological variables were ultrasound examination for fluid collection, plain film for pneumoperitoneum, and water-soluble contrast examination for extravasation. In addition, the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system and Mannheim Peritonitis Index (MPI) were used to evaluate the general condition of patient and severity of peritonitis.7
2.2. Nonoperative management
Intravenous fluid resuscitation, appropriate antibiotics, nasogastric suction, and acid-reducing pharmacotherapy (H2 antagonists or proton-pump inhibitor) were included in nonoperative management. Patients receiving nonoperative management were treated in intensive care unit. The vital and abdominal signs were rechecked by an experienced surgeon every 4 hours. Operative therapy was indicated for either progression or failure of improvement of peritonitis within 12 hours. Treatment for H. pylori began once the patients were able to tolerate oral intake. Follow-up gastroduodenoscopy was done at 4–6 weeks to monitor ulcer healing.
2.3. Statistical analysis
Continuous variables were divided into clinically meaningful categories as described earlier and compared between the two patient groups using Chi-square tests. The logistic regression analysis was used to identify variables associated with successful nonoperative treatment, as opposed to surgery therapy. All variables with univariable p < 0.2 were considered for the multivariable model. All variables with an adjusted p < 0.1 were retained in the final model. A clinical score was constructed based on the final logistic regression model, in which one point was assigned for the presence of each predictive factor.
To assess the discriminant ability of this score, a receiver operating characteristic (ROC) curve was obtained and the area under the curve (AUC) was calculated. The AUC can be interpreted as the probability that a randomly chosen patient having surgical therapy will have a higher score than a randomly chosen patient with successful nonoperative treatment. Values of 0.7 or more are often considered clinically useful.
The sensitivity and specificity of each threshold of the score were examined, as well as the likelihood ratio for successful nonoperative treatment for each value of the score. According to Bayes' theorem, the likelihood ratio represents the change in the odds of successful nonoperative treatment between pretest and post-test (post-test odds = likelihood ratio × pretest odds). A likelihood ratio of 1 indicates that the test result does not change the odds of successful nonoperative treatment, and likelihood ratios of 5 or more (alternatively, 0.2 or less) are considered to be clinically useful.8 Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Between January 2002 and December 2010, 241 patients with PPU admitted to our hospital were included in this study. There were 168 male and 73 female patients with a median age of 45 years (range: 21–92 years). Initially, 132 patients received nonoperative treatment; of these, 25 patients converted to surgery after 12 hours. Finally, 134 patients required surgical therapy. Clinical, laboratory, and radiological characteristics of the patients are summarized in Table 1. The clinical results of these two groups including length of hospital stay, expenditure, and morbidity were comparable (Table 2). However, the systemic infective complications were more common in the nonoperative treatment group than in the surgical group. The incidence of abdominal abscess and sepsis was 3.7% and 1.9%, respectively, in the nonsurgical group. In the surgical group, the incidence rate was 1.5% and 0.7%, respectively (Table 2).
| Nonoperative management (n = 107) | Surgery (n = 134) | p∗ | |
|---|---|---|---|
| Sex | 0.691 | ||
| Female | 31 | 42 | |
| Male | 76 | 92 | |
| Age ≥70 y | 0.001 | ||
| Yes | 11 | 37 | |
| No | 96 | 97 | |
| Pain duration prior to admission≥12 h | 0.064 | ||
| Yes | 16 | 33 | |
| No | 91 | 101 | |
| Fever ≥38°C | 0.058 | ||
| Yes | 42 | 69 | |
| No | 65 | 65 | |
| Steroid use | 0.172 | ||
| Yes | 2 | 7 | |
| No | 105 | 127 | |
| NSAIDs use | 0.337 | ||
| Yes | 26 | 40 | |
| No | 81 | 94 | |
| Helicobacter pylori infection | 0.430 | ||
| Yes | 65 | 88 | |
| No | 42 | 46 | |
| Satiety when perforated | 0.585 | ||
| Yes | 41 | 56 | |
| No | 66 | 78 | |
| Leukocyte count ≥12 × 109/L | 0.041 | ||
| Yes | 89 | 123 | |
| No | 18 | 11 | |
| Albumin level ≥30 g/L | 0.399 | ||
| Yes | 95 | 114 | |
| No | 12 | 20 | |
| Ultrasound: fluid collection | <0.001 | ||
| Yes | 15 | 76 | |
| No | 92 | 58 | |
| Plain film: pneumoperitoneum | 0.875 | ||
| None | 15 | 22 | |
| Unilateral | 66 | 80 | |
| Bilateral | 26 | 32 | |
| Water-soluble contrast examination: extravasation | <0.001 | ||
| Yes | 7 | 82 | |
| No | 100 | 52 | |
| APACHE II ≥8 | <0.001 | ||
| Yes | 32 | 79 | |
| No | 75 | 55 | |
| MPI ≥21 | 0.002 | ||
| Yes | 22 | 52 | |
| No | 85 | 82 |
∗Chi-square test.
APACHE II = Acute Physiology and Chronic Health Evaluation II; NSAIDs = nonsteroidal anti-inflammatory drugs; MPI = Mannheim Peritonitis Index.
| Nonoperative management (n = 107) | Surgery (n = 134) | p | |
|---|---|---|---|
| Morbidity | 15.9% (17/107) | 17.2% (23/134) | 0.791 |
| Respiratory infection | 7 (6.5) | 8 (6.0) | |
| Wound infection | 0 (0) | 8 (6.0) | |
| Wound dehiscence | 0 (0) | 3 (2.2) | |
| Abdominal abscess | 4 (3.7) | 2 (1.5) | |
| Sepsis | 2 (1.9) | 1 (0.7) | |
| Urinary infection | 2 (1.9) | 2 (1.5) | |
| Renal failure | 1 (0.9) | 1 (0.7) | |
| Cardiovascular | 1 (0.9) | 0 (0) | |
| Length of hospital stay (d) | 11.2 ± 1.3 | 10.9 ± 1.4 | 0.089 |
| Expenditure (RMB) | 11,188.3 ± 2847.4 | 10,591.9 ± 2733.4 | 0.099 |
Data are presented as n (%) or mean ± SD, unless otherwise indicated.
3.1. Comparison between the nonoperative group and the surgical group
Patients who received either nonoperative management or underwent surgical exploration with PPU were compared. Age ≥70 years, fluid collection detected by ultrasound, contrast extravasation detected by gastroduodenal imaging, and APACHE II ≥8 were significant variables in the multivariable model (Table 3). Because fever and white blood cell count are factors included in calculating the APACHE II score, we had recalculated the data after excluding them in APACHE II, and the results were similar. Age (≥70 years), positive finding for ultrasound, and water-soluble contrast examination were still the independent factors against nonoperative management. The odds ratio [95% confidence interval (CI)] value was 0.293 (0.116–0.721), 0.202 (0.047–0.325), and 0.029 (0.012–0.089), respectively, in multivariable analysis.
| Unadjusted | Adjusted | Score points | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR | p | ||
| Age ≥70 y | 0.276 (0.148–0.514) | 0 | 0.274 (0.120–0.626) | 0.002 | 1 |
| Fever ≥38°C | 0.519 (0.329–0.819) | 0.005 | Not retained | 0 | |
| Steroid use | 0.408 (0.129–1.294) | 0.128 | Not retained | 0 | |
| Leukocyte count ≥12 × 109/L | 0.421 (0.204–0.869) | 0.019 | Not retained | 0 | |
| Ultrasound: fluid collection | 0.126 (0.072–0.220) | 0 | 0.108 (0.054–0.217) | 0 | 1 |
| Water-soluble contrast examination: extravasation | 0.037 (0.016–0.084) | 0 | 0.031 (0.013–0.077) | 0 | 1 |
| APACHE II ≥8 | 0.307 (0.191–0.492) | 0 | 0.292 (0.151–0.561) | 0 | 1 |
| MPI ≥21 | 0.393 (0.237–0.654) | 0 | Not retained | 0 | |
APACHE II = Acute Physiology and Chronic Health Evaluation II; CI = confidence interval; MPI = Mannheim Peritonitis Index; OR = odds ratio.
3.2. Elaboration of a clinicoradiological score for prediction of need for surgical therapy
One point was given for one clinical criteria (age ≥70 years), each of two radiological criteria (fluid collection detected by ultrasound and contrast extravasation detected by gastroduodenal imaging), and one scoring system (APACHE II ≥8), leading to a maximum score of 4. The distribution of patients with PPU according to the predictive score and the proportion of patients with each score value who had nonoperative therapy are shown in Table 4.
| Score | No. of patients | No. of patients receiving nonoperative management | Sensitivity (%) test: considered positive if ≥score | Specificity (%) test: considered negative if <score | Likelihood ratio of successful nonoperative management at given score | Interpretation of likelihood ratio |
|---|---|---|---|---|---|---|
| 0 | 10 | 9 | 100 | 0 | ∞ | Strong evidence for nonoperative management |
| 1 | 24 | 20 | 82.0 | 2.2 | 4.5 | Weak evidence for nonoperative management |
| 2 | 32 | 15 | 42.0 | 11.1 | 0.8 | Neutral evidence (neither for nor against nonoperative management) |
| 3 | 20 | 6 | 12.0 | 48.9 | 0.4 | Moderate evidence against nonoperative management |
| 4 | 9 | 0 | 0 | 80.0 | 0 | Strong evidence against nonoperative management |
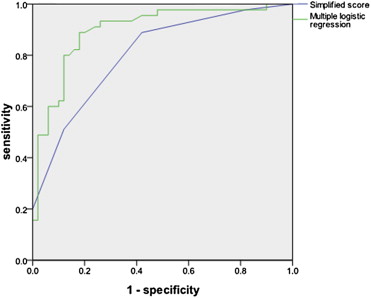
Fig. 1 shows ROC curves for the logistic regression model from Table 2 and the clinicoradiological score, for which the AUCs were 0.898 (95% CI 0.832–0.963) and 0.804 (95% CI 0.717–0.891), respectively. The loss of discriminative ability resulting from simplification of the risk equation was therefore minimal.
|
|
|
Figure 1. Receiver operating characteristic curves for the multiple logistic regression model and the simplified score. |
4. Discussion
The feasibility of nonoperative management of PPU has not been provided until the results of the first randomized controlled trial was published in 1989. Crofts et al4 showed that about 72% of the cases (29/40) can be successfully treated by nonoperative management with similar morbidity and mortality compared with the immediate surgical group.4 However, the indication of nonoperative management for PPU has not been well established. This study tried to devise a score that would help clinicians select patients with PPU who can benefit from nonoperative management. Based on univariable and multivariable analyses, four parameters were significantly correlated with the therapy approach: age ≥70 years, fluid collection detected by ultrasound, contrast extravasation detected by gastroduodenal imaging, and APACHE II ≥8. The score developed by combining these parameters proved accurate in predicting the need for surgery.
Secondary peritonitis rather than peptic ulcer itself threaten the life of patients with PPU, and therefore, the severity of peritonitis should be the major concern when deciding the treatment approach. However, our study did not include the MPI score as one of the four parameters that strongly suggested surgical therapy for PPU. A small amount of peritoneal effusion is difficult to find due to the large volume of abdominal cavity, and fluid collection detected by ultrasound prompts significant leakage of the gastrointestinal contents and severe chemical peritonitis leading to abdominal infection. The water-soluble contrast imaging is an important examination to determine whether the perforation is closed, and continuing spillover of contrast agent suggests that perforation is not closed and that gastrointestinal contents continue to leak. Positive results for the two radiological examinations reflect that peritoneal contamination is severe and aggravating, which are included in our scoring system. In addition, age and APACHE II score, which reflect the general condition of patients, are also the important parameters suggesting surgical therapy.
With the exception of contrast extravasation detected by gastroduodenal imaging, all determinants in the present score have previously been identified as independent predictors of morbidity or mortality, which could explain the reasonability of our scoring system.4; 9 ; 10 In several previous studies, duration of abdominal pain prior to admission was an independent factor significantly associating with the prognosis of PPU patients, which was not proven by our study.11 ; 12 In our study, the cutoff point of duration of abdominal pain prior to admission was 12 hours rather than 24 hours, which might lead to the exclusion of pain duration from our scoring system. The model proposed needs further validation to determine whether it can be applied to all patients with PPU. However, the AUC exceeded 0.80, indicating that this score can help in decision making. This four-point score is most useful when values are either low (0) or high (4). The intermediate values of 2 or 3 require more careful interpretation. Continued surveillance and regular reassessment are probably reasonable courses of action in these patients.
In our series, 44% of cases (107/241) received nonoperative management with the morbidity rate of 15.9%, which was comparable with the surgical group which had a morbidity rate of 17.2%. Several factors have been confirmed to be significantly associated with morbidity including pain for more than 24 hours, coexistence of major medical illnesses, amount of abdominal liquid more than 200 mL, preoperative shock, and resection surgery.9; 11; 12 ; 13 The overall mortality of our patients was 5.4% (13/241), which was lower than several previous studies.14 ; 15 In addition, our study also demonstrated that nonoperative management did not prolong the hospital stay or increase the expenditure for selected patients.
Taken together, our study showed that nonoperative management was effective and safe in selected patients with PPU. Besides, we proposed a severity model to aid in nonoperative decision making. This severity score, if validated, does not replace but may supplement individual clinical judgment. It is a method to assess quantitatively the severity of PPU and might help in monitoring the evolution of a patients condition after admission, when a nonoperative approach is initially preferred.
References
- 1 A. Christensen, R. Bousfield, J. Christiansen; Incidence of perforated and bleeding peptic ulcers before and after the introduction of H2-receptor antagonists; Ann Surg, 207 (1988), pp. 4–6
- 2 F. Sánchez-Bueno, P. Marín, A. Ríos, et al.; Has the incidence of perforated peptic ulcer decreased over the last decade?; Dig Surg, 18 (2001), pp. 444–447
- 3 H. Taylor; Perforated peptic ulcer; treated without operation; Lancet, 2 (1946), pp. 441–444
- 4 T.J. Crofts, K.G. Park, R.J. Steele, S.S. Chung, A.K. Li; A randomized trial of nonoperative treatment for perforated peptic ulcer; N Engl J Med, 320 (1989), pp. 970–973
- 5 C. Marshall, P. Ramaswamy, F.G. Bergin, I.L. Rosenberg, D.J. Leaper; Evaluation of a protocol for the non-operative management of perforated peptic ulcer; Br J Surg, 86 (1999), pp. 131–134
- 6 Y.A. Gul, M.F. Shine, F. Lennon; Non-operative management of perforated duodenal ulcer; Ir J Med Sci, 168 (1999), pp. 254–256
- 7 M.M. Linder, H. Wacha, U. Feldmann, G. Wesch, R.A. Streifensand, E. Gundlach; The Mannheim Peritonitis Index. An instrument for the intraoperative prognosis of peritonitis; Chirurg, 58 (1987), pp. 84–92 [Article in German]
- 8 D.A. Grimes, K.F. Schulz; Refining clinical diagnosis with likelihood ratios; Lancet, 365 (2005), pp. 1500–1505
- 9 J.T. Mäkelä, H. Kiviniemi, P. Ohtonen, S.O. Laitinen; Factors that predict morbidity and mortality in patients with perforated peptic ulcers; Eur J Surg, 168 (2002), pp. 446–451
- 10 F.Y. Lee, K.L. Leung, B.S. Lai, S.S. Ng, S. Dexter, W.Y. Lau; Predicting mortality and morbidity of patients operated on for perforated peptic ulcers; Arch Surg, 136 (2001), pp. 90–94
- 11 C. Noguiera, A.S. Silva, J.N. Santos, et al.; Perforated peptic ulcer: main factors of morbidity and mortality; World J Surg, 27 (2003), pp. 782–787
- 12 M.H. Møller, M.C. Engebjerg, S. Adamsen, J. Bendix, R.W. Thomsen; The peptic ulcer perforation (PULP) score: a predictor of mortality following peptic ulcer perforation. A cohort study; Acta Anaesthesiol Scand, 56 (2012), pp. 655–662
- 13 C.H. Li, W.H. Chang, S.C. Shih, S.C. Lin, M.J. Bair; Perforated peptic ulcer in southeastern Taiwan; J Gastroenterol Hepatol, 25 (2010), pp. 1530–1536
- 14 M.H. Møller, S. Adamsen, R.W. Thomsen, A.M. Møller; Peptic Ulcer Perforation (PULP) trial group. Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation; Br J Surg, 98 (2011), pp. 802–810
- 15 D.L. Buck, M. Vester-Andersen, M.H. Møller; Accuracy of clinical prediction rules in peptic ulcer perforation: an observational study; Scand J Gastroenterol, 47 (2012), pp. 28–35
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?