(Created page with " ==Summary== The overall incidence of cardiac tumor is very low, and malignant cardiac tumors account for only 25% of all cardiac tumors. Angiosarcomas are the most common t...") |
m (Scipediacontent moved page Draft Content 581332609 to Byeon et al 2014a) |
(No difference)
| |
Latest revision as of 11:30, 26 May 2017
Summary
The overall incidence of cardiac tumor is very low, and malignant cardiac tumors account for only 25% of all cardiac tumors. Angiosarcomas are the most common type of malignant cardiac tumors, characterized by rapidly proliferating, extensively infiltrating anaplastic cells derived from blood vessels and lining irregular blood-filled spaces. We present a 26-year-old man with angiosarcoma involving the right atrium, which was misdiagnosed as aortic intramural hematoma by computed tomography, finally confirmed by transesophageal echocardiography during the operation.
Keywords
angiosarcoma;cardiac tamponade;hematoma;transesophageal echocardiography
1. Introduction
Primary cardiac tumors are rare, with an incidence at autopsy ranging from 0.0017% to 0.033%, and most of them are benign. Malignant cardiac tumors only account for 25% of all cardiac tumors. Angiosarcomas are the most common type of malignant cardiac tumors in adults.1 At present, transesophageal echocardiography (TEE) is regarded as a useful tool in determining tumor size and anatomical localization with high sensitivities of up to 97%, although computed tomography (CT) and magnetic resonance imaging (MRI) have excellent diagnostic advantages with regard to tumor delineation and spread.2
We present a 26-year-old man with primary right atrial angiosarcoma—which was initially misdiagnosed as aortic intramural hematoma (IMH) by CT—that was finally confirmed by TEE during the operation.
2. Case Report
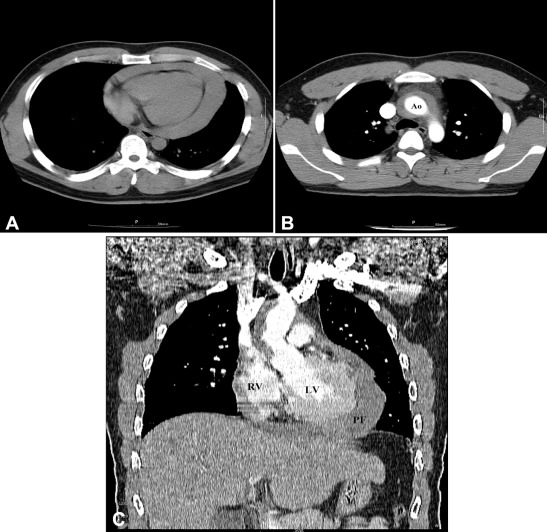
A 26-year-old man was who transferred to our hospital was diagnosed to have aortic IMH with cardiac tamponade. On admission, the patient showed confused mentality and heavy sweating. His initial vital signs were as follows: systolic blood pressure, 60–70 mmHg; heart rate, 130–140 beats/minute; peripheral oxygen saturation, 70–75%. The jugular vein was engorged, but hepatomegaly or audible cardiac murmurs were not detected. A chest X-ray showed cardiomegaly with a cardiothoracic ratio of 58%. An electrocardiogram (ECG) showed sinus tachycardia with a heart rate of 128 beats/minute and T abnormality of inferior leads. A chest CT scan previously taken from another hospital revealed IMH and pericardial effusion without any evidence of abnormal mass in chest structure (Fig. 1).
|
|
|
Figure 1. Chest computed tomography findings. (A) Unenhanced scan shows large pericardial fluid collection with computed tomography density measurements consistent with hemopericardium. (B) Enhanced axial scan shows aortic intramural hematoma without any evidence of abnormal mass in chest structure. (C) Enhanced coronal view shows large pericardial fluid collection and aortic intramural hematoma. Ao = aorta; LV = left ventricle; PF = pericardial fluid; RV = right ventricle. |
Further imaging studies such as transthoracic echocardiography (TTE), ECG gating cardiac CT, or MRI could not be conducted because of poor compliance and unstable vital signs. An emergency operation for the resection of IMH and hemiarch replacement of the ascending aorta was planned although aortic IMH contained only in the ascending aorta without any distal extension is rare in young healthy individuals.
During the anesthetic induction, arterial cannulation for monitoring of arterial blood pressure was performed on both radial arteries. Anesthesia was intravenously induced with 0.3 mg/kg etomidate and 0.6 mg/kg rocuronium. After tracheal intubation, anesthesia was maintained by 1–2 vol.% of sevoflurane and 0.5–20 μg/kg/minute of remifentanil. After induction, vital signs showed consistent hypotension (60–70 mmHg of systolic blood pressure), tachycardia (140–160 beats/minute of heart rate), and hypoxia (70–75% of peripheral oxygen saturation). Inotropic and vasoactive drugs such as norepinephrine (0.1–0.5 μg/kg/minute), epinephrine (0.01–0.05 μg/kg/minute), dobutamine (2–5 μg/kg/minute), and vasopressin (0.0003–0.001 units/kg/minute) were infused.
Because the patients vital signs were unstable, the cardiac surgeon performed peripheral cannulation for an emergent cardiopulmonary bypass prior to sternotomy to rapidly stabilize circulation and facilitate surgery for thoracic aorta dissection. An 18F arterial cannula and a 22F venous cannula were inserted through the right femoral artery and vein under TEE guidance.
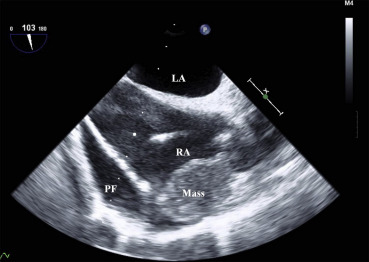
TEE revealed massive pericardial effusion over the right heart and a redundant motion of the right atrial free wall, and showed a 3.5 × 2.5 cm mass with bright echodensity in the lateral wall of the right atrium adjacent to the superior vena cava (Fig. 2). Moreover, after sternotomy, the ascending aorta showed no surgical pathology. Then, the surgical plan was changed for a resection of the right atrial mass. For the right atrial mass excision, another 24F venous cannula was inserted at the superior vena cava and antegrade cold blood cardioplegia was infused via a root cannula for myocardial protection.
|
|
|
Figure 2. Transesophageal echocardiography reveals massive pericardial fluid collection over the right heart and redundant motion of the right atrial free wall and shows a 3.5 × 2.5 cm mass in the lateral wall of the right atrium. LA = left atrium; PF = pericardial fluid; RA = right atrium. |
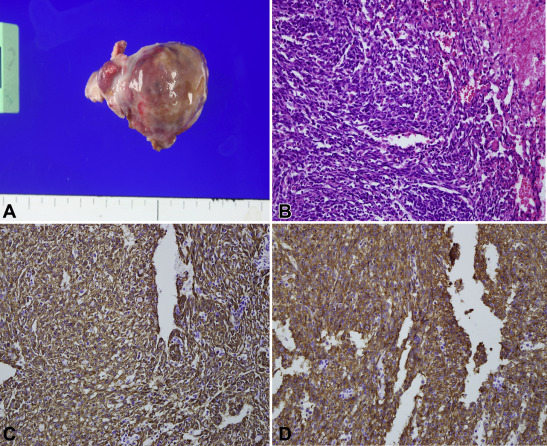
After the mass resection, the right atrium was repaired with a bovine pericardial patch. The patient was uneventfully weaned from cardiopulmonary bypass and recovered without any neurological sequelae. The cardiopulmonary bypass time and aortic cross clamping time was 92 minutes and 45 minutes, respectively. After the operation, he was transferred to the intensive care unit. He was extubated on postoperative day (POD) 1, ambulated slowly on POD 2, and discharged on POD 6. The postoperative pathological findings showed a 3.6 × 3.0 × 1.8 cm angiosarcoma. Immunohistochemical staining was positive for CD34 and CD31, confirming vascular differentiation and supporting the diagnosis of a poorly differentiated cardiac angiosarcoma (Fig. 3). He then underwent adjuvant radiotherapy and chemotherapy 3 weeks after the operation.
|
|
|
Figure 3. Pathological findings. (A) Gross finding shows a 3.6 × 3.0 × 1.8 cm mass. (B) The tumor cells are spindled and ovoid and reveal slit-like and interconnecting vascular spaces on hematoxylin and eosin stain. (C, D) The tumor cells show immunopositivity for CD31 and CD34 immunohistochemical staining. |
3. Discussion
Cardiac angiosarcoma is characterized by rapidly proliferating, extensively infiltrating anaplastic cells derived from blood vessels and lining irregular blood-filled spaces.3 Several diagnostic tools for primary cardiac tumors are available. CT is a useful technique for the diagnosis of angiosarcomas; however, non-ECG-gated chest CT can sometimes lead to a missed diagnosis of angiosarcoma that looks so thin because of the artifact of cardiac movement.4 Nevertheless, chest CT and MRI can reveal infiltrative growth and the extracardiac extent of sarcomas, which are criteria that help us distinguish benign from malignant lesions and assess resectability.5 Echocardiography is a screening modality, showing tumor size, location, mobility, and attachment. The diagnostic sensitivities of TTE and TEE are 93.3% and 97%, respectively. TEE better depicts the posterior wall of the left atrium, atrial septum, right heart, and descending aorta.6
Moreover, intraoperative TEE can delineate the undiagnosed cardiac pathology by CT or MRI, which could be a useful tool to modify a surgical plan that has already been determined. Sheikh et al7 reported that TEE contributes to improve 6–17% of surgical outcomes by allowing surgeons to make changes to the traditional method of surgery. Consequently, in our case, the surgical plan was changed from ascending aorta replacement to resection of cardiac mass with pericardial patch by assisted intraoperative TEE.
Although the clinical manifestation may be vague and may depend on the location of the tumor, cardiac angiosarcomas commonly present with cardiac tamponade with hemodynamic instability; thus, pericardiocentesis could be a valuable technique to reduce hemodynamic derangement prior to surgery.8 However, the patient was transferred without any delay and we performed peripheral cannulation for the concept of venoarterial extracorporeal membrane oxygenation rather than pericardiocentesis. If the vital signs can be maintained with medical support, we believe this peripheral cannulation strategy has considerable advantages such as rapid stabilization of circulation and facilitation of the surgical procedure involving the thoracic aorta.
Cardiac angiosarcoma is a serious diagnosis because of its highly metastatic potential and poor prognosis, and should be included in the differential diagnosis of nontraumatic bloody pericardial effusions, even with negative early investigations.9 In conclusion, intraoperative TEE could be a valuable tool in diagnosing and localizing cardiac tumors, even those masked by an artifact of cardiac movement in conventional non-ECG gating chest CT.
Informed consent
This case report was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 05-2012-052).
Acknowledgments
This work was supported by a clinical research grant from Pusan National University Yangsan Hospital.
References
- 1 A.P. Burke, D. Cowan, R. Virmani; Primary sarcomas of the heart; Cancer, 69 (1992), pp. 387–395
- 2 Q. Meng, H. Lai, J. Lima, W. Tong, Y. Qian, S. Lai; Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases; Int J Cardiol, 84 (2002), pp. 69–75
- 3 S.K. Ohri, P. Nihoyannopoulos, K.M. Taylor, B.E. Keogh; Angiosarcoma of the heart causing cardiac rupture: a rare cause of hemopericardium; Ann Thorac Surg, 55 (1993), pp. 525–528
- 4 M. Inoko, K. Iga, K. Kyo, et al.; Primary cardiac angiosarcoma detected by magnetic resonance imaging but not by computed tomography; Intern Med, 40 (2001), pp. 391–395
- 5 W.B. Dawson, J.R. Mayo, N.L. Muller; Computed tomography of cardiac and pericardial tumors; Can Assoc Radiol J, 41 (1990), pp. 270–275
- 6 D.N. Smith, K. Shaffer, E.F. Patz; Imaging features of nonmyxomatous primary neoplasms of the heart and pericardium; Clin Imaging, 22 (1998), pp. 15–22
- 7 K.H. Sheikh, N.P. de Bruijn, J.S. Rankin, et al.; The utility of transesophageal echocardiography and doppler color flow imaging in patients undergoing cardiac valve surgery; J Am Coll Cardiol, 15 (1990), pp. 363–372
- 8 L.F. Hsu, C. Scavée, P. Jaïs, M. Hocini, M. Haïssaguerre; Transcardiac pericardiocentesis: an emergency life-saving technique for cardiac tamponade; J Cardiovasc Electrophysiol, 14 (2003), pp. 1001–1003
- 9 E. Chalhoub, B.I. Mattar, W. Shaheen, T.K. Schulz; Cardiac angiosarcoma presenting with tamponade; Intern Med, 51 (2012), pp. 2905–2907
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?