(Created page with "==Abstract== The QT interval on the human electrocardiogram is normally in the order of 450 ms, and reflects the summated durations of action potential (AP) depolarization a...") |
m (Scipediacontent moved page Draft Content 219701113 to Tse et al 2016a) |
(No difference)
| |
Latest revision as of 10:49, 19 May 2017
Abstract
The QT interval on the human electrocardiogram is normally in the order of 450 ms, and reflects the summated durations of action potential (AP) depolarization and repolarization of ventricular myocytes. Both prolongation and shortening in the QT interval have been associated with ventricular tachy-arrhythmias, which predispose affected individuals to sudden cardiac death. In this article, the molecular determinants of the AP duration and the causes of long and short QT syndromes (LQTS and SQTS) are explored. This is followed by a review of the recent advances on their arrhythmogenic mechanisms involving reentry and/or triggered activity based on experiments conducted in mouse models. Established and novel clinical risk markers based on the QT interval for the prediction of arrhythmic risk and cardiovascular mortality are presented here. It is concluded by a discussion on strategies for the future rational design of anti-arrhythmic agents.
Keywords
Cardiac arrhythmia;Repolarization;Long QT syndrome;Short QT syndrome;Wavelength
1. Introduction
Long and short QT syndromes (LQTS and SQTS) are primary electrical disorders of the heart that predispose the affected individuals to sudden cardiac death via the development of malignant ventricular arrhythmias. Both syndromes can arise congenitally from ion channel mutations, or can have acquired causes. In this article, the ionic basis of the QT interval is examined, summarizing recent advances into the electrophysiological mechanisms of arrhythmogenesis of both LQTS and SQTS.
1.1. The QT interval
The QT interval of the human electrocardiogram (ECG) is a marker of the duration of the cellular action potential (AP) [1]. It varies with heart rate, and therefore a correction must be made before its interpretation. Different formulae have been proposed for this purpose (Table 1). The commonest is Bazetts formula, given by the QT interval divided by the square root of the RR interval. However, this method overestimates QT interval at high heart rates and underestimates it at low heart rates [2]. By contrast, Fridericia formula, in which QT interval is divided by the cubic root of the RR interval, works better for slow heart rates. Other methods include the Framingham and Hodges formulae. The upper limit of a normal corrected QT (QTc) interval by Bazetts formula is 440 ms for males and 460 ms for females. The latest European Society of Cardiology guideline produced in 2015 suggests upper and lower limits of 480 ms and 360 ms, respectively, for both males and females [3]. The QT interval increases with age and long QT interval is commonly associated with electrolyte abnormalities [4], drugs [5]; [6] ; [7], medical conditions such as epilepsy and diabetes mellitus [8] ; [9]. The risk of arrhythmogenesis is increased at both extremes of the QT interval. To understanding why this is the case, the ionic determinants of the AP and the mechanisms by which their alterations lead to repolarization abnormalities must be considered.
| QT correction method | Formula |
|---|---|
| Bazett | QT/RR1/2 |
| Fridericia | QT/RR1/3 |
| Framingham | QT + 0.154 (1000 − RR) |
| Hodges | QT + 105 (1/RR − 1) |
1.2. Inward and outward currents determine the duration of the ventricular APs
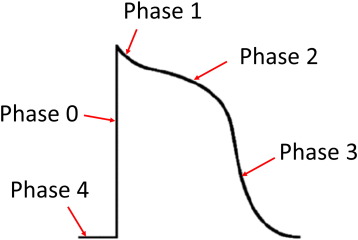
Generation of the ventricular APs is dependent upon voltage-gated conductances, and AP durations are determined by the balance between inward and outward currents. An AP has five phases: fast upstroke (phase 0) followed by a spike (phase 1) and plateau (phase 2) morphology, and further repolarization (phase 3), where the transmembrane voltage returns to the resting membrane potential (phase 4) (Fig. 1). Phase 0 is mediated by voltage-gated Na+ channels with rapid activation and inactivation kinetics. Phase 1 involves rapid repolarization mediated by the fast and slow transient outward K+ currents, Ito,f and Ito,s, respectively. Phase 3 is maintained by competing inward currents mediated by the voltage-gated L-type Ca2 + channel (ICa,L) and Na+-Ca2 + exchanger (INCX), and outward currents mediated by the voltage-gated delayed rectifier K+ channels (IK) [10]. Phase 3 can be explained by a high driving force for K+ efflux due to a large potential difference between the membrane potential and the K+ equilibrium potential. Phase 4 is the resting membrane potential at − 80 and − 64 mV [11]; [12] ; [13], which is set by the inward rectifier current, IK1 with contribution from the weak inward rectifying ATP-dependent K+ channels (IK,ATP) [14]. The QT interval includes the durations of both ventricular depolarization and repolarization. Importantly, the end of repolarization (action potential duration, APD) usually coincides with the resumption of tissue excitability (effective refractory period, ERP).
|
|
|
Fig. 1. Morphology of the human ventricular action potential. Phase 0 is the action potential upstroke mediated by Na+ channel activation. Phase 1 represents early rapid repolarization due to transient outward K+ currents. Phase 2 is the plateau phase determined by a balance between inward Ca2 + and outward K+ currents. Phase 3 is late repolarization attributed to delayed rectifier K+ currents, bringing the membrane potential back to the baseline (phase 4). |
1.3. Long QT syndromes (LQTS)
Long QT syndromes (LQTS) is characterized by an abnormally long QT interval of ≥ 450 ms on the ECG. The first hereditary long QT syndrome was discovered by Jervell and Lange-Nielsen (JLN) in 1957 [15]. In this family, the parents had normal QT intervals and hearing, producing six children. Four suffered from both long QT interval and congenital sensorineural deafness and the remaining two were normal. Three of these four children suffered from sudden death. JLN syndrome was later shown to have an autosomal recessive inheritance. In the 1960s, Romano and Ward separately reported families suffering from QT prolongation but normal hearing, and the syndrome, after whom is named, has an autosomal dominant inheritance [16].
LQTS is caused by a decrease in repolarizing currents or an increase in depolarizing currents, with either congenital or acquired causes. Today, thirteen genetic LQTS subtypes have been identified thus far. Loss-of-function mutations in the different types of K+ channels are responsible for LQTS types 1 (KCNQ1), 2 (KCNH2), 5 (KCNE1), 6 (KCNE2), 7 (KCNJ2) and 13 (KCNJ5). By contrast, gain-of-function mutations in Na+ channel subunits lead to LQTS types 3 (SCN5A) and 10 (SCN4B), and in the L-type Ca2 + channel produces LQT type 8 (CACNA1C, Timothy syndrome). Mutations in supporting proteins are responsible for the LQT type 4 (ANKB), 9 (CAV3), 11 (AKAP9) and 12 (SNTA1) phenotypes. Recent studies have implicated calmodulin mutations in patients suffering from a LQTS with previously unidentified genetic causes [17]; [18] ; [19]. Moreover, a long QT phenotype has been implicated in sudden unexpected death in epilepsy (SUDEP), caused by increased late Na+ current (INa, L) mediated by neuronal Na+ channel isoforms [20] ; [21].
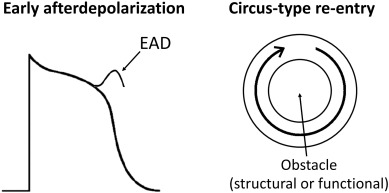
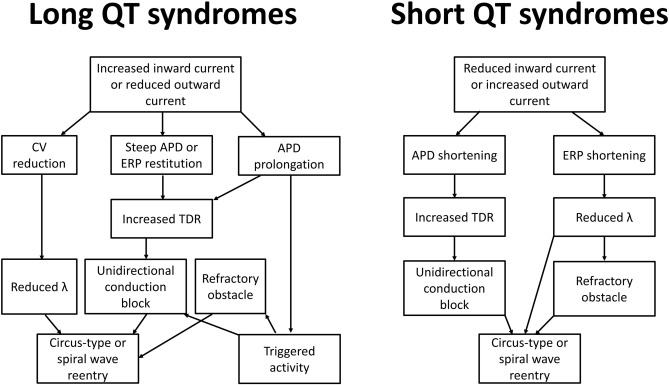
By contrast, acquired causes of LQTS are much more common than genetic causes. These are commonly due to electrolyte abnormalities, most frequently hypokalaemia. A hypokalaemia mouse model has been used to study the arrhythmogenic mechanisms of LQTS, demonstrating several consequences of APD prolongation (Table 2). Firstly, it increases the Ca2 + current available Na+ channel reactivation during the repolarizing phase, leading to the development of early afterdepolarizations and subsequent triggered activity (Fig. 2) [22]. Secondly, AP prolongation preferentially occurs at the epicardium compared to the endocardium, resulting in an increase in the transmural dispersion of repolarization (TDR) [22]. Reduced ERP of the ventricular myocardium [23] and unaltered conduction velocity (CV) were observed, leading to a decrease in excitation wavelength (λ) given by CV x ERP. λ is the path length that is occupied by the action potential wave. Theoretically, a smaller λ can more easily support a re-entrant circuit, thereby increasing the likelihood of reentrant arrhythmias (Fig. 3). In congenital long QT syndromes, the ERP is not typically altered. Similarly, CV is not reduced unless the specific mutation produces loss-of-function mutations in Na+ channels, which may give rise to overlapping phenotype of LQTS with Brugada syndrome and conduction defect [24]; [25]; [26] ; [27]. Moreover, the emergence of APD alternans, attributed to increased steepness of APD restitution together with the abnormal repolarization gradient can lead to unidirectional conduction block and thereby reentry [28] ; [29].
| Abnormalities | LQTS | SQTS | References |
|---|---|---|---|
| Molecular mechanisms | Increased inward currents or reduced outward currents | Reduced inward currents or reduced outward currents | [15] ; [30] |
| Triggered activity | Early afterdepolarizations from LTCC reactivation | Not observed | [86] |
| Substrates for reentry | |||
| CV | ↔/↓ | ↔/↑ | [63] |
| APD | ↑ | ↓ | [53] |
| ERP | ↑ | ↓ | [63] |
| TDR | ↑ | ↑ | [54] ; [87] |
| λ | ↓ | ↓ | [37] ; [63] |
| APD alternans | ↑ | N/A | [88] ; [89] |
| CV restitution | ↔ | N/A | [29]; [90] ; [91] |
| APD restitution | ↑ gradient | N/A | [29]; [92] ; [93] |
| VERP restitution | ↑ gradient | N/A | [94] |
|
|
|
Fig. 2. Early afterdepolarizations can produce triggered activity, which can initiate arrhythmias. Circus-type reentry requires slowed conduction, unidirectional conduction block and a central obstacle around which the action potential wave can circulate. |
|
|
|
Fig. 3. Arrhythmogenic mechanisms in long and short QT syndromes. Prolongation in action potential duration (APD) can predispose to the development of early afterdepolarizations, in turn producing triggered activity that can serve to initiate arrhythmias. Reentrant substrates may involve alterations in conduction velocity (CV), prolongation or shortening of APD or shortening of effective refractory period (ERP). These abnormalities can in turn lead to increased transmural dispersion of repolarization (TDR), which can promote unidirectional conduction block and an obstacle around which the action potential can circulate. Together with reduced wavelength (λ = CV × ERP), these can increase the susceptibility of tachycardia by circus-type or spiral wave reentry. |
1.4. Short QT syndromes (SQTS)
Short QT syndrome (SQTS) is characterized by an abnormally short QT interval of < 350 ms on the ECG. It predisposes affected individuals to an increased risk of atrial and ventricular arrhythmias, in particular ventricular fibrillation, and is therefore an important cause of sudden cardiac death [30]. Shortening of QT interval reflects accelerated repolarization, which can result from increased activity of repolarizing currents, or decreased activity of depolarizing currents. SQTS, like LQTS, can have congenital or acquired causes. Six genetic subtypes of SQTS have been identified thus far. Gain-of-function mutations in the K+ channel genes, KCNH2, KCNQ1 [31] ; [32] and KCNJ2 [33] are responsible for SQT types 1, 2 and 3, respectively. By contrast, loss-of-function mutations in L-type Ca2 + channel subunits, CACNA1C, CACNB2 and CACNA2D1, are found in SQT types 4, 5 and 6, respectively [34]. Interestingly, some patients diagnosed with Brugada syndrome have demonstrated shortened QT intervals [34]. This is perhaps not surprising upon consideration of the molecular mechanisms involved, because loss-of-function mutations in the inward currents, which tips the net current in the outward direction, are observed in both SQTS and Brugada syndrome. Acquired causes are more common, including electrolyte abnormalities of hyperkalaemia or hypercalcaemia, myocardial ischaemia, acidosis or carnitine deficiency [35] ; [36]. Hyperthermia can also cause a shortened QT interval, as can drugs such as digitalis, acetylcholine, catecholamines or KATP activators. Short QT intervals have also been associated with epilepsy, particularly during the ictal and post-ictal states [8].
The mechanism of arrhythmogenesis in SQTS is less well-understood than that of LQTS. Recent work in mice demonstrated shortening in ERP in concert with APD [37], leading to decreased λ and a higher risk of circus-type reentry. Abnormal APD restitution leading to APD alternans is unlikely to play a role in SQTS because only long diastolic intervals are engaged where the restitution curve is flat, unlike the case of LQTS where it was possible to engage the steep portion of the restitution curve at low diastolic intervals [28]. CV may be increased due to ERP shortening, but this would not be expected to be pro-arrhythmic since this would increase rather than decrease λ [38]. The similarities and differences of the electrophysiological consequences of LQTS and SQTS are detailed in Table 1.
1.5. Arrhythmic risk prediction: markers based on repolarization and QT interval
Pre-clinical models have been useful for the studying the mechanisms of cardiac arrhythmogenesis and provide a platform for testing the arrhythmogenic potential of drugs [29]; [37]; [39]; [40]; [41]; [42]; [43]; [44]; [45]; [46] ; [47]. Experiments in these systems have demonstrated different arrhythmic risk markers, such as increased TDR given by the maximum APD difference across the myocardial wall [48], increased critical interval for re-excitation given by the APD-ERP difference [23]; [49]; [50] ; [51], shortened λ and reduced λ-TRIAD (which is based on λ and repolarization properties of triangulation, reverse use dependence, instability and dispersion: TRIaD) [52]. The clinical marker traditionally used for predicting arrhythmic risk has been QTc[53]. However, its lack of accuracy led to the development of other markers [52], such as QT dispersion (QTd) [54] ; [55], interval from the peak to the end of the T wave [56] (Tpeak – Tend, reflecting increased TDR [57]), and (Tpeak – Tend)/QT ratio [58].
However, none of the above repolarization markers takes into account action potential conduction, yet λ, which incorporates both processes, is an important determinant of arrhythmic risk [59]; [60] ; [61]. Three novel markers based on conduction-repolarization have been proposed thus far. The first is the index of Cardiac Electrophysiological Balance (iCEB: QT/QRS) proposed by Lu and colleagues, which is a surrogate marker of λ [62]. Indeed, this has demonstrated utility in predicting arrhythmic risk in drug-induced settings, LQTS and Brugada syndrome [63]. The other two markers, (Tpeak–Tend)/QRS and (Tpeak–Tend)/(QT × QRS), have recently been put forward by Tse, but these remain to be validated clinically. Tses indices are based on the observations that both conduction and repolarization abnormalities are important in arrhythmogenesis and Tpeak–Tend was a significant predictor of SCD even after adjusting for, inter alia, QTc and QRS durations [64]. They may therefore provide superior predictive values for arrhythmic risk than the repolarization markers discussed above and even iCEB [65] ; [66]. Tses indices were subsequently modified by Tse and Yan to incorporate QRS dispersion (QRSd) reflecting CV dispersion, yielding QRSd × (Tpeak–Tend)/QRS, and QRSd × (Tpeak–Tend)/(QRS × QT) [67]. It was proposed that the term QRSd/QRS could be a surrogate marker of CV dispersion coefficient based on the standard deviation of the mean CV [68]. Other non-invasive methods of assessing the function of the heart include magnetocardiography, which may provide additional insights into risk stratification in the future [69]; [70]; [71]; [72]; [73]; [74]; [75] ; [76].
1.6. Therapeutic strategies
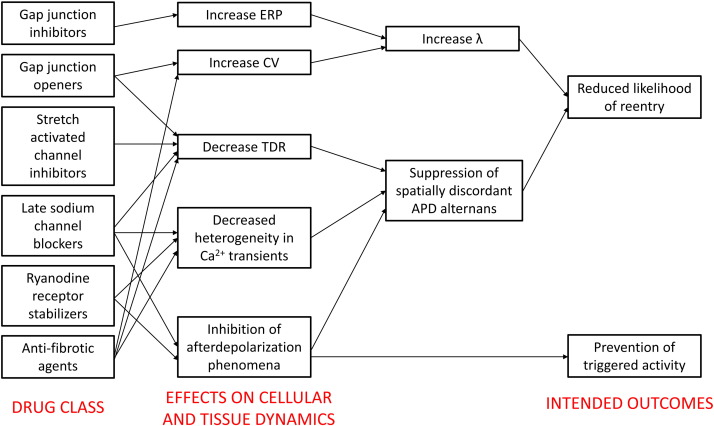
For LQTS, beta blockers are only effective in preventing ventricular tachycardia in approximately 70% of the patients. The remaining 30% are susceptible to arrhythmias. For SQTS, quinidine or disopyramide are recommended. In both syndromes, definitive treatment is implantable cardioverter-defibrillator (ICD) insertion. There is therefore a need to develop more effective agents for anti-arrhythmic therapy. A better understanding of the mechanisms of arrhythmogenesis would allow rational drug design that aims to reverse the electrophysiological abnormalities in question. Application of pre-clinical results to clinical medicine could result in effective translation for the benefit of patients, which is illustrated by the following two examples that demonstrate important proofs-of-concept. Firstly, hypokalaemia modelling LQTS produces AP prolongation, reduced ERP, reduced λ, increased TDR, increased APD restitution slopes and increased amplitude of APD alternans. Gap junction inhibition using heptanol normalized ERP and therefore λ without correcting for the remaining repolarization abnormalities [29]. Secondly, hyperkalaemia modelling SQTS results in shortened APD and ERP, reduced λ and increased TDR. Anti-arrhythmic effects of hypercalcaemia were associated with reversal of ERP changes and normalization of λ, again without correcting for the repolarization abnormalities [37]. Together, the above studies demonstrate that prolonging myocardial refractoriness with an aim of increasing λ is a viable strategy. Other approaches that have demonstrated some success in pre-clinical models are increasing ERP or CV, decreasing heterogeneities in CV, APD, ERP or Ca2 + transients, or suppressing after depolarization phenomena (Fig. 4: modified from Tse et al. with permission [28]). Novel agents using such strategies are gap junction inhibitors [77]; [78]; [79] ; [80] and openers [81] ; [82], stretch-activated channel modulators, late sodium channel blockers [83], ryanodine receptor stabilizers [84] and anti-fibrotic agents [85]. It is likely that a systems physiology approach will play a large role in studying the complex spatial and temporal properties of cardiac dynamics. Its application will no doubt transform arrhythmia management by identifying agents that have lower toxicity and toxic side effects of currently available drugs.
|
|
|
Fig. 4. Future drug classes for anti-arrhythmic therapy based on rational drug design: gap junction inhibitors, gap junction openers, stretch-activated channel inhibitors, late sodium channel blockers, ryanodine receptor stabilizers and anti-fibrotic agents. Adapted from Tse et al. (2016) with permission [28]. |
Conflict of interest
None declared.
Acknowledgements
GT received a BBSRC Doctoral Training Award at the University of Cambridge and thanks the Croucher Foundation of Hong Kong for supporting his clinical assistant professorship. YC is supported by the ESRC for her PhD studies.
References
- [1] S. Weidmann; Effect of current flow on the membrane potential of cardiac muscle; J. Physiol., 115 (2) (1951), pp. 227–236
- [2] H.C. Bazett; An analysis of the time-relations of electrocardiograms; Ann. Noninvasive Electrocardiol., 2 (2) (1997), pp. 177–194
- [3] S.G. Priori, C. Blomström-Lundqvist, A. Mazzanti, N. Blom, M. Borggrefe, J. Camm, P.M. Elliott, D. Fitzsimons, R. Hatala, G. Hindricks, P. Kirchhof, K. Kjeldsen, K.-H. Kuck, A. Hernandez-Madrid, N. Nikolaou, T.M. Norekvål, C. Spaulding, D.J. Van Veldhuisen, P. Kolh, G.Y.H. Lip, S. Agewall, G. Barón-Esquivias, G. Boriani, W. Budts, H. Bueno, D. Capodanno, S. Carerj, M.G. Crespo-Leiro, M. Czerny, C. Deaton, D. Dobrev, Ç. Erol, M. Galderisi, B. Gorenek, T. Kriebel, P. Lambiase, P. Lancellotti, D.A. Lane, I. Lang, A.J. Manolis, J. Morais, J. Moreno, M.F. Piepoli, F.H. Rutten, B. Sredniawa, J.L. Zamorano, F. Zannad; 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death; Eur. Heart J., 36 (41) (2015), pp. 2793–2867
- [4] D.B. Diercks, G.M. Shumaik, R.A. Harrigan, W.J. Brady, T.C. Chan; Electrocardiographic manifestations: electrolyte abnormalities; Am. J. Emerg. Med., 27 (2) (2004), pp. 153–160
- [5] D.M. Roden, K.A. Thompson, B.F. Hoffman, R.L. Woosley; Clinical features and basic mechanisms of quinidine-induced arrhythmias; J. Am. Coll. Cardiol., 8 (1 Suppl A) (1986), pp. 73A–78A
- [6] W. Haverkamp, G. Breithardt, A.J. Camm, M.J. Janse, M.R. Rosen, C. Antzelevitch, D. Escande, M. Franz, M. Malik, A. Moss, R. Shah; The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology; Cardiovasc. Res., 47 (2) (2000), pp. 219–233
- [7] R.R. Fenichel, M. Malik, C. Antzelevitch, M. Sanguinetti, D.M. Roden, S.G. Priori, J.N. Ruskin, R.J. Lipicky, L.R. Cantilena; Drug-induced torsades de pointes and implications for drug development; J. Cardiovasc. Electrophysiol., 15 (4) (2004), pp. 475–495
- [8] R. Surges, P. Taggart, J.W. Sander, M.C. Walker; Too long or too short? New insights into abnormal cardiac repolarization in people with chronic epilepsy and its potential role in sudden unexpected death; Epilepsia, 51 (5) (2010), pp. 738–744
- [9] M. Veglio, M. Borra, L.K. Stevens, J.H. Fuller, P.C. Perin; The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM complication study group; Diabetologia, 42 (1) (1999), pp. 68–75
- [10] E. Carmeliet; Cardiac ionic currents and acute ischemia: from channels to arrhythmias; Physiol. Rev., 79 (3) (1999), pp. 917–1017
- [11] S.N. Hatem, A. Coulombe, E. Balse; Specificities of atrial electrophysiology: clues to a better understanding of cardiac function and the mechanisms of arrhythmias; J. Mol. Cell. Cardiol., 48 (1) (2010), pp. 90–95
- [12] G.J. Amos, E. Wettwer, F. Metzger, Q. Li, H.M. Himmel, U. Ravens; Differences between outward currents of human atrial and subepicardial ventricular myocytes; J. Physiol., 491 (Pt 1) (1996), pp. 31–50
- [13] G.A. Peeters, M.C. Sanguinetti, Y. Eki, H. Konarzewska, D.G. Renlund, S.V. Karwande, W.H. Barry; Method for isolation of human ventricular myocytes from single endocardial and epicardial biopsies; Am. J. Phys., 268 (4 Pt 2) (1995), pp. H1757–H1764
- [14] A. Noma; ATP-regulated K+ channels in cardiac muscle; Nature, 305 (5930) (1983), pp. 147–148
- [15] A. Jervell, F. Lange-Nielsen; Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death; Am. Heart J., 54 (1) (1957), pp. 59–68
- [16] C. Romano, G. Gemme, R. Pongiglione; Rare cardiac arrythmias of the pediatric age. Ii. Syncopal attacks due to paroxysmal ventricular fibrillation. (Presentation of 1st case in Italian pediatric literature); Clin. Pediatr., 45 (1963), pp. 656–683 (Bologna)
- [17] L. Crotti, C.N. Johnson, E. Graf, G.M. De Ferrari, B.F. Cuneo, M. Ovadia, J. Papagiannis, M.D. Feldkamp, S.G. Rathi, J.D. Kunic, M. Pedrazzini, T. Wieland, P. Lichtner, B.-M. Beckmann, T. Clark, C. Shaffer, D.W. Benson, S. Kääb, T. Meitinger, T.M. Strom, W.J. Chazin, P.J. Schwartz, A.L. George; Calmodulin mutations associated with recurrent cardiac arrest in infants; Circulation, 127 (9) (2013), pp. 1009–1017
- [18] N. Makita, N. Yagihara, L. Crotti, C.N. Johnson, B.-M. Beckmann, M.S. Roh, D. Shigemizu, P. Lichtner, T. Ishikawa, T. Aiba, T. Homfray, E.R. Behr, D. Klug, I. Denjoy, E. Mastantuono, D. Theisen, T. Tsunoda, W. Satake, T. Toda, H. Nakagawa, Y. Tsuji, T. Tsuchiya, H. Yamamoto, Y. Miyamoto, N. Endo, A. Kimura, K. Ozaki, H. Motomura, K. Suda, T. Tanaka, P.J. Schwartz, T. Meitinger, S. Kääb, P. Guicheney, W. Shimizu, Z.A. Bhuiyan, H. Watanabe, W.J. Chazin, A.L. George; Novel calmodulin mutations associated with congenital arrhythmia susceptibility; Circ. Cardiovasc. Genet., 7 (4) (2014), pp. 466–474
- [19] G.J. Reed, N.J. Boczek, S.P. Etheridge, M.J. Ackerman; CALM3 mutation associated with long QT syndrome; Heart Rhythm., 12 (2) (2015), pp. 419–422
- [20] M. Biet, N. Morin, M. Lessard-Beaudoin, R.K. Graham, S. Duss, J. Gagne, N.T. Sanon, L. Carmant, R. Dumaine; Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ Current: potential mechanism for sudden death in epilepsy; Circ. Arrhythm. Electrophysiol., 8 (4) (2015), pp. 912–920
- [21] A.L. George Jr.; Misplaced brain sodium channels in heart kindle sudden death in epilepsy; Circ. Arrhythm. Electrophysiol., 8 (4) (2015), pp. 769–771
- [22] M.J. Killeen, G. Thomas, I.S. Gurung, C.A. Goddard, J.A. Fraser, M.P. Mahaut-Smith, W.H. Colledge, A.A. Grace, C.L. Huang; Arrhythmogenic mechanisms in the isolated perfused hypokalaemic murine heart; Acta Physiol (Oxford), 189 (1) (2007), pp. 33–46
- [23] I.N. Sabir, J.A. Fraser, M.J. Killeen, A.A. Grace, C.L. Huang; The contribution of refractoriness to arrhythmic substrate in hypokalaemic Langendorff-perfused murine hearts; Pflugers Arch., 454 (2007), pp. 209–222
- [24] R. Surber, S. Hensellek, D. Prochnau, G.S. Werner, K. Benndorf, H.R. Figulla, T. Zimmer; Combination of cardiac conduction disease and long QT syndrome caused by mutation T1620K in the cardiac sodium channel; Cardiovasc. Res., 77 (4) (2008), pp. 740–748
- [25] C. Bezzina, M.W. Veldkamp, M.P. van den Berg, A.V. Postma, M.B. Rook, J.-W. Viersma, I.M. van Langen, G. Tan-Sindhunata, M.T.E. Bink-Boelkens, A.H. van der Hout, M.M.A.M. Mannens, A.A.M. Wilde; A single Na+ channel mutation causing both long-QT and Brugada syndromes; Circ. Res., 85 (12) (1999), pp. 1206–1213
- [26] I. Rivolta, H. Abriel, M. Tateyama, H. Liu, M. Memmi, P. Vardas, C. Napolitano, S.G. Priori, R.S. Kass; Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes; J. Biol. Chem., 276 (33) (2001), pp. 30623–30630
- [27] C. Veltmann, H. Barajas-Martinez, C. Wolpert, M. Borggrefe, R. Schimpf, R. Pfeiffer, G. Cáceres, E. Burashnikov, C. Antzelevitch, D. Hu; Further insights in the most common SCN5A mutation causing overlapping phenotype of long QT syndrome, Brugada syndrome, and conduction defect; J. Am. Heart Assoc., 5 (7) (2016)
- [28] G. Tse, S.T.T. Wong, V.Y.T. Lee, H.Y. Lin, J.M. Yeo; Cardiac dynamics: alternans and arrhythmogenesis; J Arrhythm., 12 (2016), pp. 1–10
- [29] G. Tse, S.T. Wong, V. Tse, J.M. Yeo; Restitution analysis of alternans using dynamic pacing and its comparison with S1S2 restitution in heptanol-treated, hypokalaemic Langendorff-perfused mouse hearts; Biomed. Rep., 4 (2016), pp. 673–680
- [30] R. Brugada, K. Hong, R. Dumaine, J. Cordeiro, F. Gaita, M. Borggrefe, T.M. Menendez, J. Brugada, G.D. Pollevick, C. Wolpert, E. Burashnikov, K. Matsuo, Y.S. Wu, A. Guerchicoff, F. Bianchi, C. Giustetto, R. Schimpf, P. Brugada, C. Antzelevitch; Sudden death associated with short-QT syndrome linked to mutations in HERG; Circulation, 109 (1) (2004), pp. 30–35
- [31] C. Bellocq, A.C. van Ginneken, C.R. Bezzina, M. Alders, D. Escande, M.M. Mannens, I. Baro, A.A. Wilde; Mutation in the KCNQ1 gene leading to the short QT-interval syndrome; Circulation, 109 (20) (2004), pp. 2394–2397
- [32] R. Schimpf, C. Wolpert, F. Gaita, C. Giustetto, M. Borggrefe; Short QT syndrome; Cardiovasc. Res., 67 (3) (2005), pp. 357–366
- [33] S.G. Priori, S.V. Pandit, I. Rivolta, O. Berenfeld, E. Ronchetti, A. Dhamoon, C. Napolitano, J. Anumonwo, M.R. di Barletta, S. Gudapakkam, G. Bosi, M. Stramba-Badiale, J. Jalife; A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene; Circ. Res., 96 (7) (2005), pp. 800–807
- [34] C. Antzelevitch, G.D. Pollevick, J.M. Cordeiro, O. Casis, M.C. Sanguinetti, Y. Aizawa, A. Guerchicoff, R. Pfeiffer, A. Oliva, B. Wollnik, P. Gelber, E.P. Bonaros Jr., E. Burashnikov, Y. Wu, J.D. Sargent, S. Schickel, R. Oberheiden, A. Bhatia, L.F. Hsu, M. Haissaguerre, R. Schimpf, M. Borggrefe, C. Wolpert; Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death; Circulation, 115 (4) (2007), pp. 442–449
- [35] A.J. Workman, I. MacKenzie, B.J. Northover; A K(ATP) channel opener inhibited myocardial reperfusion action potential shortening and arrhythmias; Eur. J. Pharmacol., 419 (1) (2001), pp. 73–83
- [36] J. Roussel, F. Labarthe, J. Thireau, F. Ferro, C. Farah, J. Roy, M. Horiuchi, M. Tardieu, B. Lefort, J. Francois Benoist, A. Lacampagne, S. Richard, J. Fauconnier, D. Babuty, J.Y. Le Guennec; Carnitine deficiency induces a short QT syndrome; Heart Rhythm., 13 (1) (2015), pp. 165–174
- [37] G. Tse, B. Sun, S.T. Wong, V. Tse, J.M. Yeo; Ventricular anti-arrhythmic effects of hypercalcaemia treatment in hyperkalaemic, Langendorff-perfused mouse hearts; Biomed. Rep., 5 (3) (2016), pp. 301–310
- [38] G. Tse; Mechanisms of cardiac arrhythmias; J. Arrhythm., 32 (2) (2015), pp. 75–81 http://doi.org/10.1016/j.joa.2015.11.003
- [39] G. Tse, S.T. Wong, V. Tse, J.M. Yeo; Monophasic action potential recordings: which is the recording electrode?; J. Basic Clin. Physiol. Pharmacol., 27 (5) (2016), pp. 457–462
- [40] G. Tse, S.T. Wong, V. Tse, J.M. Yeo; Depolarization vs. repolarization: what is the mechanism of ventricular arrhythmogenesis underlying sodium channel haploinsufficiency in mouse hearts?; Acta Physiol (Oxford), 218 (4) (2016), pp. 234–235
- [41] G. Tse, T.H. Lai, J.M. Yeo, V. Tse, S.H. Wong; Mechanisms of electrical activation and conduction in the gastrointestinal system: lessons from cardiac electrophysiology; Front. Physiol., 7 (2016), p. 182
- [42] G. Tse, E.T. Lai, V. Tse, J.M. Yeo; Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus; J. Diabetes Res., 2016 (2016), Article 2848759
- [43] G. Tse, E.T. Lai, Y.W. Chan, J.M. Yeo, B.P. Yan; What is the arrhythmic substrate in viral myocarditis? Insights from clinical and animal studies; Front. Physiol., 7 (2016), p. 308
- [44] Z. Chen, B. Sun, G. Tse, J. Jiang, W. Xu; Reversibility of both sinus node dysfunction and reduced HCN4 mRNA expression level in an atrial tachycardia pacing model of tachycardia-bradycardia syndrome in rabbit hearts; Int. J. Clin. Exp. Pathol., 9 (8) (2016), pp. 8526–8531
- [45] G. Tse, S.T. Wong, V. Tse, J.M. Yeo; Determination of action potential wavelength restitution in Scn5a+/− mouse hearts modelling human Brugada syndrome; J. Geriatr. Cardiol. (2016) (accepted for publication)
- [46] G. Tse, E.T. Lai, J.M. Yeo, B.P. Yan; Electrophysiological mechanisms of Bayés syndrome: insights from clinical and mouse studies; Front. Phys., 7 (2016), p. 188
- [47] L. Choy, J.M. Yeo, V. Tse, S.P. Chan, G. Tse; Cardiac disease and arrhythmogenesis: mechanistic insights from mouse models; Int. J. Cardiol. Heart Vasc., 12 (2016), pp. 1–10
- [48] S. Sicouri, S. Moro, S. Litovsky, M.V. Elizari, C. Antzelevitch; Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart; J. Cardiovasc. Electrophysiol., 8 (11) (1997), pp. 1269–1279
- [49] O.E. Osadchii, S.P. Olesen; Electrophysiological determinants of hypokalaemia-induced arrhythmogenicity in the Guinea-pig heart; Acta Physiol (Oxford), 197 (4) (2009), pp. 273–287
- [50] O.E. Osadchii; Mechanisms of hypokalemia-induced ventricular arrhythmogenicity; Fundam. Clin. Pharmacol., 24 (5) (2010), pp. 547–559
- [51] O.E. Osadchii; Dofetilide promotes repolarization abnormalities in perfused Guinea-pig heart; Cardiovasc. Drugs Ther., 26 (6) (2012), pp. 489–500
- [52] L.M. Hondeghem; QTc prolongation as a surrogate for drug-induced arrhythmias: fact or fallacy?; Acta Cardiol., 66 (6) (2011), pp. 685–689
- [53] B. Surawicz; The QT interval and cardiac arrhythmias; Annu. Rev. Med., 38 (1987), pp. 81–90
- [54] H. Elming, E. Holm, L. Jun, C. Torp-Pedersen, L. Kober, M. Kircshoff, M. Malik, J. Camm; The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens; Eur. Heart J., 19 (9) (1998), pp. 1391–1400
- [55] N.J. Linker, P. Colonna, C.A. Kekwick, J. Till, A.J. Camm, D.E. Ward; Assessment of QT dispersion in symptomatic patients with congenital long QT syndromes; Am. J. Cardiol., 69 (6) (1992), pp. 634–638
- [56] Y. Xia, Y. Liang, O. Kongstad, M. Holm, B. Olsson, S. Yuan; Tpeak–Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine; J. Interv. Card. Electrophysiol., 14 (2) (2005), pp. 79–87
- [57] P. Gupta, C. Patel, H. Patel, S. Narayanaswamy, B. Malhotra, J.T. Green, G.X. Yan; T(p–e)/QT ratio as an index of arrhythmogenesis; J. Electrocardiol., 41 (6) (2008), pp. 567–574
- [58] J. Castro Hevia, C. Antzelevitch, F. Tornes Barzaga, M. Dorantes Sanchez, F. Dorticos Balea, R. Zayas Molina, M.A. Quinones Perez, Y. Fayad Rodriguez; Tpeak–Tend and Tpeak–Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome; J. Am. Coll. Cardiol., 47 (9) (2006), pp. 1828–1834
- [59] G. Tse, S.T. Wong, V. Tse, J.M. Yeo; Variability in local action potential durations, dispersion of repolarization and wavelength restitution in aged wild-type and Scn5a+/− mouse hearts modelling human Brugada syndrome; J. Geriatr. Cardiol. (2016) (accepted for publication)
- [60] Y.C. Hsieh, S.F. Lin, T.C. Lin, C.T. Ting, T.J. Wu; Therapeutic hypothermia (30 °C) enhances arrhythmogenic substrates, including spatially discordant alternans, and facilitates pacing-induced ventricular fibrillation in isolated rabbit hearts; Circ. J., 73 (12) (2009), pp. 2214–2222
- [61] O.E. Osadchii; Impact of hypokalemia on electromechanical window, excitation wavelength and repolarization gradients in Guinea-pig and rabbit hearts; PLoS One, 9 (8) (2014), Article e105599
- [62] H.R. Lu, G.-X. Yan, D.J. Gallacher; A new biomarker – index of cardiac electrophysiological balance (iCEB) – plays an important role in drug-induced cardiac arrhythmias: beyond QT-prolongation and Torsades de Pointes (TdPs); J. Pharmacol. Toxicol. Methods, 68 (2) (2013), pp. 250–259
- [63] T. Robyns, H.R. Lu, D.J. Gallacher, C. Garweg, J. Ector, R. Willems, S. Janssens, D. Nuyens; Evaluation of index of cardio-electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk; Ann. Noninvasive Electrocardiol., 21 (3) (2016), pp. 294–304
- [64] R. Panikkath, K. Reinier, A. Uy-Evanado, C. Teodorescu, J. Hattenhauer, R. Mariani, K. Gunson, J. Jui, S.S. Chugh; Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death; Circ. Arrhythm. Electrophysiol., 4 (4) (2011), pp. 441–447
- [65] G. Tse; Novel conduction-repolarization indices for the stratification of arrhythmic risk; J. Geriatr. Cardiol., 13 (2016), pp. 811–812
- [66] G. Tse; (Tpeak–Tend)/QRS and (Tpeak–Tend)/(QT × QRS): novel markers for predicting arrhythmic risk in Brugada syndrome; Europace (2016) http://doi.org/10.17863/CAM.113 (in press)
- [67] G. Tse, B.P. Yan; Novel arrhythmic risk markers incorporating QRS dispersion: QRSd × (Tpeak–Tend)/QRS and QRSd × (Tpeak–Tend)/(QT × QRS); Ann. Noninvasive Electrocardiol. (2016) http://doi.org/10.1111/anec.12397 (accepted for publication)
- [68] M. Boulaksil, S.K. Winckels, M.A. Engelen, M. Stein, T.A. van Veen, J.A. Jansen, A.C. Linnenbank, M.F. Bierhuizen, W.A. Groenewegen, M.F. van Oosterhout, J.H. Kirkels, N. de Jonge, A. Varró, M.A. Vos, J.M. de Bakker, H.V. van Rijen; Heterogeneous Connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias; Eur. J. Heart Fail., 12 (9) (2010), pp. 913–921
- [69] G. Tse, A. Ali, F. Alpendurada, S. Prasad, C.E. Raphael, V. Vassiliou; Tuberculous constrictive pericarditis; Res. Cardiovasc. Med., 4 (4) (2015), Article e29614
- [70] G. Tse, A. Ali, S.K. Prasad, V. Vassiliou, C.E. Raphael; Atypical case of post-partum cardiomyopathy: an overlap syndrome with arrhythmogenic right ventricular cardiomyopathy?; BJR | case reports, 1 (2) (2015), p. 20150182
- [71] V. Vassiliou, C. Chin, A. Perperoglou, G. Tse, A. Ali, C. Raphael, A. Jabbour, D. Newby, D. Pennell, M. Dweck, S. Prasad; 93 Ejection fraction by cardiovascular magnetic resonance predicts adverse outcomes post aortic valve replacement; Heart, 100 (Suppl. 3) (2014), pp. A53–A54
- [72] J.S. Kwong, B. Leithauser, J.W. Park, C.M. Yu; Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: a review of clinical data; Int. J. Cardiol., 167 (5) (2013), pp. 1835–1842
- [73] U. Steinhoff, S. Knappe-Grueneberg, A. Schnabel, L. Trahms, F. Smith, P. Langley, A. Murray, H. Koch; Magnetocardiography for pharmacology safety studies requiring high patient throughput and reliability; J. Electrocardiol., 37 (2004), pp. 187–192 (Suppl.)
- [74] K. Yoshida, K. Ogata, T. Inaba, Y. Nakazawa, Y. Ito, I. Yamaguchi, A. Kandori, K. Aonuma; Ability of magnetocardiography to detect regional dominant frequencies of atrial fibrillation; J. Arrhythm., 31 (6) (2015), pp. 345–351
- [75] Y. Ito, K. Shiga, K. Yoshida, K. Ogata, A. Kandori, T. Inaba, Y. Nakazawa, Y. Sekiguchi, H. Tada, K. Sekihara, K. Aonuma; Development of a magnetocardiography-based algorithm for discrimination between ventricular arrhythmias originating from the right ventricular outflow tract and those originating from the aortic sinus cusp: a pilot study; Heart Rhythm., 11 (9) (2014), pp. 1605–1612
- [76] Y. Sato, K. Yoshida, K. Ogata, T. Inaba, H. Tada, Y. Sekiguchi, Y. Ito, T. Ishizu, Y. Seo, I. Yamaguchi, A. Kandori, K. Aonuma; An increase in right atrial magnetic strength is a novel predictor of recurrence of atrial fibrillation after radiofrequency catheter ablation; Circ. J., 76 (7) (2012), pp. 1601–1608
- [77] R. Veeraraghavan, J. Lin, G.S. Hoeker, J.P. Keener, R.G. Gourdie, S. Poelzing; Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study; Pflugers Arch., 467 (10) (2015), pp. 2093–2105
- [78] S.A. George, K.J. Sciuto, J. Lin, M.E. Salama, J.P. Keener, R.G. Gourdie, S. Poelzing; Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene; Pflugers Arch., 467 (11) (2015), pp. 2287–2297
- [79] R. Veeraraghavan, R.G. Gourdie, S. Poelzing; Mechanisms of cardiac conduction: a history of revisions; Am. J. Physiol. Heart Circ. Physiol., 306 (5) (2014), pp. H619–H627
- [80] R. Veeraraghavan, S. Poelzing, R.G. Gourdie; Old cogs, new tricks: a scaffolding role for connexin43 and a junctional role for sodium channels?; FEBS Lett., 588 (8) (2014), pp. 1244–1248
- [81] Y.C. Hsieh, J.C. Lin, C.Y. Hung, C.H. Li, S.F. Lin, H.I. Yeh, J.L. Huang, C.P. Lo, K. Haugan, B.D. Larsen, T.J. Wu; Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts; Heart Rhythm., 13 (1) (2015), pp. 251–261
- [82] L. Ruan, X. Quan, L. Li, R. Bai, M. Ni, R. Xu, C. Zhang; Increasing gap junction coupling suppresses ibutilide-induced torsades de pointes; Exp. Ther. Med., 7 (5) (2014), pp. 1279–1284
- [83] A.S. Alves Bento, D. Bacic, J. Saran Carneiro, B.D. Nearing, H. Fuller, F.A. Justo, S. Rajamani, L. Belardinelli, R.L. Verrier; Selective late INa inhibition by GS-458967 exerts parallel suppression of catecholamine-induced hemodynamically significant ventricular tachycardia and T-wave alternans in an intact porcine model; Heart Rhythm., 12 (12) (2015), pp. 2508–2514
- [84] S.E. Lehnart, C. Terrenoire, S. Reiken, X.H.T. Wehrens, L.-S. Song, E.J. Tillman, S. Mancarella, J. Coromilas, W.J. Lederer, R.S. Kass, A.R. Marks; Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias; Proc. Natl. Acad. Sci. U. S. A., 103 (20) (2006), pp. 7906–7910
- [85] R.A. Bathgate, E.D. Lekgabe, J.T. McGuane, Y. Su, T. Pham, T. Ferraro, S. Layfield, R.D. Hannan, W.G. Thomas, C.S. Samuel, X.J. Du; Adenovirus-mediated delivery of relaxin reverses cardiac fibrosis; Mol. Cell. Endocrinol., 280 (1–2) (2008), pp. 30–38
- [86] C.T. January, J.M. Riddle; Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2 + current; Circ. Res., 64 (1989), pp. 977–990
- [87] P.M. Okin, R.B. Devereux, B.V. Howard, R.R. Fabsitz, E.T. Lee, T.K. Welty; Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: the strong heart study; Circulation, 101 (1) (2000), pp. 61–66
- [88] R.L. Verrier, J. Sroubek; Quantitative T-wave alternans analysis for sudden cardiac death risk assessment and guiding therapy: answered and unanswered questions: for: proceedings of ICE2015 Comandatuba, Brazil, sudden death symposium; J. Electrocardiol., 49 (3) (2016), pp. 429–438
- [89] R.L. Verrier, T. Ikeda; Ambulatory ECG-based T-wave alternans monitoring for risk assessment and guiding medical therapy: mechanisms and clinical applications; Prog. Cardiovasc. Dis., 56 (2) (2013), pp. 172–185
- [90] S. Mironov, J. Jalife, E.G. Tolkacheva; Role of conduction velocity restitution and short-term memory in the development of action potential duration alternans in isolated rabbit hearts; Circulation, 118 (1) (2008), pp. 17–25
- [91] I. Banville, R.A. Gray; Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias; J. Cardiovasc. Electrophysiol., 13 (11) (2002), pp. 1141–1149
- [92] W.B. Nicolson, G.P. McCann, P.D. Brown, A.J. Sandilands, P.J. Stafford, F.S. Schlindwein, N.J. Samani, G.A. Ng; A novel surface electrocardiogram-based marker of ventricular arrhythmia risk in patients with ischemic cardiomyopathy; J. Am. Heart Assoc., 1 (4) (2012), Article e001552
- [93] W.B. Nicolson, G.P. McCann, M.I. Smith, A.J. Sandilands, P.J. Stafford, F.S. Schlindwein, N.J. Samani, G.A. Ng; Prospective evaluation of two novel ECG-based restitution biomarkers for prediction of sudden cardiac death risk in ischaemic cardiomyopathy; Heart, 100 (23) (2014), pp. 1878–1885
- [94] J.M. Cao, Z. Qu, Y.H. Kim, T.J. Wu, A. Garfinkel, J.N. Weiss, H.S. Karagueuzian, P.S. Chen; Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties; Circ. Res., 84 (11) (1999), pp. 1318–1331
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?