(Created page with "==Abstract== ====Objective==== A bicuspid aortic valve (BAV) is associated with accelerated aortic valve disease (AVD) and abnormalities in aortic elasticity. We investigate...") |
m (Scipediacontent moved page Draft Content 167873532 to Petrini et al 2016a) |
(No difference)
| |
Latest revision as of 10:46, 19 May 2017
Abstract
Objective
A bicuspid aortic valve (BAV) is associated with accelerated aortic valve disease (AVD) and abnormalities in aortic elasticity. We investigated the intima-media thickness of the descending aorta (AoIMT) in patients with AVD with or without an ascending aortic aneurysm (AscAA), in relation to BAV versus tricuspid aortic valve (TAV) phenotype, type of valve disease, cardiovascular risk factors, and single-nucleotide polymorphisms (SNPs) with a known association with carotid IMT.
Methods and results
368 patients (210 with BAV, 158 with TAV,); mean age 64 ± 13 years) were examined using transesophageal echocardiography (TEE) before valvular and/or aortic surgery. No patient had a coronary disease (CAD). The AoIMT was measured on short-axis TEE images of the descending aorta using a semi-automated edge-detection technique. AoIMT was univariately (P < 0.05) related to age, blood pressure, smoking, creatinine, highly sensitive C-reactive protein, HDL, valve hemodynamics and BAV. In the TAV subgroup it was also associated with the rs200991 SNP. Using multivariate regression analysis, age was the main determinant for AoIMT (P < 0.001), followed by male gender (P = 0.02), BAV was no longer a significant predictor of AoIMT. AoIMT was still related to the rs200991 SNP in TAV (P = 0.034), and to creatinine in BAV (P = 0.019), when other variables were accounted for.
Conclusions
Intima-media thickness of the descending aorta is not affected by aortic valve morphology (BAV/TAV); age is the main determinant of AoIMT. Genetic markers (SNPs) known to influence IMT in the carotid artery seem to correlate to IMT in the descending aorta only in patients with TAV.
Keywords
Intima-media thickness;Descending aorta;Genetics;Bicuspid aortic valve;Aortic stenosis;Aortic regurgitation
1. Introduction
A bicuspid aortic valve (BAV) is prone to accelerated valve calcification leading to premature aortic valve disease (AoVD) and is associated with altered elastic properties [1]; [2] ; [3], aneurysms (AscAA) [4] ; [5], and dissection of the ascending aorta [6]. Wall changes in the ascending aorta correlating to the flow jet pattern in BAV patients with AoVD have been described [7]. Although the disturbances in flow in BAV have been demonstrated in the entire aorta, including the descending aorta [8], there are only a scarce number of studies on descending aortic function and morphology [9]; [10] ; [11].
Intima-media thickness (IMT) in the descending aorta (AoIMT) has been studied in relation to coronary artery disease (CAD) and stroke [12]. Non-laminar flow has been shown to affect the IMT, triggering migration of smooth muscle cells and monocytes as well as causing intimal hyperplasia [13] ; [14]. To our knowledge, there are no reports examining the AoIMT in patients with a BAV versus those with a tricuspid aortic valve (TAV), or its relation to different hemodynamic environments due to different aortic valve and aortic pathologies. Therefore, we examined the relationship between AoIMT and aortic valve phenotype (BAV/TAV) and type of AoVD. We also tested its possible relation to some single-nucleotide polymorphisms (SNPs) recently reported to influence carotid intima-media thickness (CIMT) [15].
2. Methods
2.1. Study population
The initial population comprised 400 patients included in the Advanced Study of Aortic Pathology (ASAP), which is a prospective single-center study performed at the Karolinska University Hospital, Stockholm, Sweden. Consecutive patients aged 18 years or over with AoVD and/or an AscAA that required elective surgery were recruited, provided they were willing to participate and free of CAD according to coronary angiography. Prior to surgery, transesophageal echocardiography (TEE) was performed on 386 patients under general anesthesia. Segments with aortic plaque were excluded, why 8 patients could not be analyzed. Further, 9 patients were excluded due to suboptimal image quality while the AoIMT could be measured in 369 patients, constituting 96% of the TEE sample. Characteristics of different subgroups of patients included in this study were described previously [11]. On inclusion, patients answered a questionnaire to assess cardiovascular risk factors. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured, and blood samples were taken for biochemical analyses. The mean blood pressure (MBP) was calculated as MBP = DBP + 1/3 (SBP–DBP). Classification of the valve phenotype was made by visual inspection of the valve intra-operatively by the surgeon. Three cusps and three commissures signified a TAV; two cusps and two commissures -with or without a raphe - signified a BAV. This information was missing in one case, so 368 patients were included in the final subgroup analysis. Each subject gave written consent for participation in the study, which was approved by the Regional Ethics Review Board, Stockholm, Sweden.
2.2. Transthoracic echocardiography (TTE)
All but three patients underwent TTE before surgery, using a Philips iE33 ultrasound scanner (Philips Medical Systems, Bothell, WA, USA). Doppler and two-dimensional echocardiography measurements were performed according to the current standards (ASE 2005, 2009) [16] ; [17]. Aortic regurgitation (AR) was graded as 0–3 (0, none; 1, mild; 2, moderate; 3, severe) according to the 2003 ASE guidelines [18], the PISA method was not used for classification. The patients were classified according to findings on TTE into four groups: aortic stenosis (AS), AR, AscAA, or with combined disease (MIX). Three patients could not perform TTE and therefore could not be classified preoperatively by this study protocol due to logistics. The lesion was defined as AS when the peak gradient (Pmax) > 50 mm Hg and mean gradient (Pmean) > 40 mm Hg, and/or aortic valve area < 1 cm2. AR was defined as an AR grade = 3 not fulfilling the AS criteria. AscAA was defined as an ascending aortic diameter of 45 or 50 mm (BAV or TAV groups, respectively) and not fulfilling the criteria for AS or AR. The MIX group consisted of patients with disease, which did not fulfill the criteria for AS, AR, or AscAA above, based on the results of TTE performed within the study protocol [19]. In patients being allocated to the MIX group TTE results differed from the primary evaluation mostly due to the fact that additional diagnostic modalities have been incorporated or different examination conditions influenced the results at the time of clinical decision making regarding cardiac surgery.
2.3. Transesophageal echocardiography
TEE was performed in the operating room with the patient under general anesthesia before surgery using a Sequoia c512 ultrasound scanner (Siemens Medical Systems, Mountain View, CA, USA) with transducer frequency of 6 or 7 MHz, mean frame rate 70 Hz. The ECG was recorded on the ultrasound images. Scanning of the descending aorta was performed in short-axis view at three predefined distances from the teeth (30, 35, 40 cm), with the 35 cm level approximately representing the level of the left atrium in the majority of patients as described previously [11]. The gain settings were adjusted to obtain an optimal quality of the image. From the three levels, we selected the level (preferably 35 cm) with the best-defined intima-media interface for measurement. Aortic valve morphology (BAV/TAV) using TEE images was determined according to the classification of Sievers and Schmidtke [20].
All the TTE and TEE examinations were read by highly experienced physicians working at the echo lab. For the TTE, all exams were divided among a group of 4 physicians. The TEE images were interpreted and IMT measurements were performed by one single reader.
2.4. Intima-media thickness of descending aorta
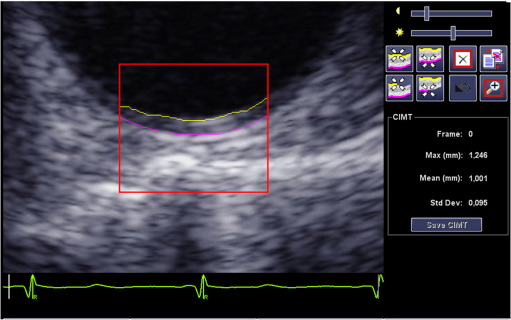
Measurements of AoIMT were performed off-line using a Syngo Arterial Health Package semi-automated edge-detection program (version 3.5; Siemens Medical Systems), allowing detection of the echogenic lines of the intima-media complex in a 10-mm-wide segment that could be adjusted to the curvature of the vessel in short axis (Fig. 1).
|
|
|
Fig. 1. Intima-media thickness of the descending aorta (AoIMT) measurement in a short-axis view in diastole. Mean value of AoIMT measured by a semi-automated edge-detection software has been reported in this study. |
The best-quality end-diastolic image with good perpendicular alignment was selected and a region of interest (ROI) was placed manually over the far wall of the descending aorta in short-axis view. The ROI length was set shorter than 10 mm in a small number of patients because of suboptimal image quality. Tracings of the intima-blood and media-adventitia borders were adjusted manually when needed. The mean AoIMT was measured from two consecutive heart beats.
2.5. Reproducibility
The intra- and interobserver variabilities of measurements were determined for AoIMT variables in 27 patients. For intraobserver variability, the same observer repeated the measurements after 1 week, whereas interobserver variability was assessed from measurements performed by two experienced independent observers. The intra- and interobserver variabilities for AoIMT expressed as a coefficient of variation (CV%) in this study were 11% and 10%, respectively.
2.6. Blood analyses
The plasma concentrations of creatinine, highly sensitive C-reactive protein (hs-CRP), HDL, LDL and serum concentrations of cholesterol and triglycerides were estimated by the Karolinska University laboratory using standard methods (Unicel DXC 800 Synchron; Beckman Coulter, Brea, CA, USA).
2.7. Single nucleotide polymorphism analysis
Genotypes were extracted from previously described genotyping using the Illumina Human 610W-Quad BeadArrays (Illumina Inc., San Diego, CA, USA) at the SNP technology platform at Uppsala University [21]. In 338 patients, we studied the rs200991 SNP in the HIST1H2BN locus and the rs4888378 SNP in the BCAR1-CFDP1-TMEM170A locus, identified previously as determinants of carotid IMT [15]. The patients in whom SNP analysis were performed were evenly spread throughout the study. In some cases the analysis was not possible due to logistical reasons. The Genome Studio software from Illumina was used for genotype calling and quality control.
2.8. Statistical analyses
Analyses were performed using commercially available SPSS software (version 22; IBM Corp., Armonk, NY, USA). Data are presented as mean ± SD or medians and (25–75th percentiles). For comparisons between groups, Students t test and analysis of variance (ANOVA) were used. Skewed variables were log transformed before analyses. Tukeys test for post hoc analysis was performed in conjunction with ANOVA. The Chi-squared test was used to analyze nominal or categorical variables. Correlations between the variables were estimated using Pearsons product–moment correlation coefficient (r). Univariate and stepwise forward multivariate regression analyses were used to evaluate the predictors of AoIMT. Colinearity was analyzed to assess the relationships between the variables included in the models. P < 0.05 was considered significant. In multivariate analysis, determinants of AoIMT with a classification cutoff of P < 0.10 were presented in the model.
3. Results
The clinical characteristics and echocardiographic data according to valve morphology are shown in Table 1. There were no differences in weight, body surface area, cholesterol, LDL, HDL, angina, heart failure, stroke/TIA, diabetes, smoking habits or ejection faction between BAV and TAV patients. In all, 366 patients were classified according to the TTE study protocol (Table 2). TAV patients displayed a thicker AoIMT within respective valve pathology compared to BAV, but TAV patients were significantly older (AS 10 years, P < 0.001; AR 12 years, P < 0.001) data not shown). In the AscAA and MIX groups there were no significant differences in AoIMT between the valve phenotype subgroups, but these constituted fewer patients. The age difference between TAV and BAV patients was significant in the AscAA group (TAV 8 years older, P < 0.02) but not in the MIX group, data not shown.
| All n = 368 | BAV n = 210 | TAV n = 158 | P-value BAV vs. TAV | |
|---|---|---|---|---|
| Age (years) | 64.3 ± 12.6 | 60.2 ± 12.2 | 69.5 ± 11.0 | < 0.001 |
| Height (m) | 1.74 ± 0.10 | 1.75 ± 0.09 | 1.72 ± 0.10 | 0.001 |
| BMI (kg/m2) | 26.9 ± 4.4 | 26.3 ± 4.2 | 27.5 ± 4.7 | < 0.05 |
| Inclusion SBP (mmHg) | 141 ± 20 | 139 ± 20 | 144 ± 20 | < 0.01 |
| Inclusion DBP (mmHg) | 80 ± 13 | 81 ± 12 | 77 ± 13 | 0.001 |
| Mean BP (mmHg) | 100 ± 12 | 101 ± 12 | 100 ± 13 | ns |
| Creatinine (μml/L) | 81 (70–91) | 80 (69–89) | 82 (70–92) | ns |
| hsCRP (mg/L) | 1.5 (0.6–3.1) | 1.1 (0.5–2.9) | 1.8 (0.8–3.6) | 0.001 |
| Triglycerides (mml/L) | 1.0 (0.8–1.5) | 1.0 (0.8–1.6) | 1.0 (0.7–1.5) | ns |
| Male gender | 245 (67%) | 148 (71%) | 97 (61%) | ns |
| Myocardial infarction | 20 (5%) | 6 (3%) | 14 (9%) | < 0.05 |
| Hypertension | 195 (53%) | 102 (49%) | 93 (59%) | < 0.05 |
| Echocardiographic data | ||||
| AoIMT (mm) | 1.37 ± 0.33 | 1.30 ± 0.33 | 1.44 ± 0.31 | < 0.001 |
| LVEDD (mm) | 52 ± 9 | 52 ± 9 | 52 ± 10 | ns |
| Pmax (mmHg) | 58 ± 37 | 62 ± 38 | 52 ± 26 | < 0.01 |
| AVA (cm2) | 1.6 ± 1.2 | 1.5 ± 1.1 | 1.7 ± 1.3 | < 0.05 |
| AR grade: 0/1/2/3 (%) | (23/44/12/21) | (24/44/13/18) | (20/44/12/25) | ns |
| Aorta ascendens/BSA (mm/m2) | 20 ± 5 | 20 ± 6 | 21 ± 5 | ns |
| Aorta descendens (mm) | 24 ± 3 | 23 ± 3 | 25 ± 3 | 0.001 |
| Aorta descendens/BSA (mm/m2) | 12 ± 2 | 12 ± 2 | 13 ± 2 | 0.001 |
BAV, bicuspid aortic valve; BMI, body mass index; hsCRP, high sensitivity C-reactive protein; TAV, tricuspid aortic valve; AoIMT, Aortic intima-media thickness; AVA, aortic valve area; LVEDD, left ventricular end-diastolic diameter; Pmax, aortic valve peak gradient; Values are expressed as mean ± SD, median and (25th–75th percentile) or as n (%) or %.
| BAV AoIMT (mm) | TAV AoIMT (mm) | P-value BAV vs. TAV | |
|---|---|---|---|
| AS n = 203 | 1.36 ± 0.31 | 1.53 ± 0.29 | < 0.001 |
| AR n = 77 | 1.10 ± 0.25 | 1.26 ± 0.23 | < 0.01 |
| AscAA n = 55 | 1.36 ± 0.36 | 1.54 ± 0.47 | ns |
| MIX n = 31 | 1.21 ± 0.29 | 1.35 ± 0.29 | ns |
AS, aortic stenosis; AR, aortic regurgitation; AscAA ascending aortic aneurysm; MIX, Mixed pathology; mean ± SD.
3.1. Genetic associations
Table 3 shows AoIMT values according to the alleles in SNPs. We only found significant differences between the genotype groups for the rs200991 SNP. There were very few patients with an AA nucleotide allele pair configuration, as expected according to Hardy–Weinberg equilibrium [22]. On post hoc analysis between groups, a non-significant trend (P = 0.098) towards a thicker AoIMT was noted in patients with the CC configuration. In a subgroup analysis of men and women, there were no significant differences in the AoIMT values between genotypes in the SNPs (data not shown). When the BAV and TAV groups were analyzed separately, only the patients with a TAV showed a difference between the rs200991 alleles. Patients with a CC allele configuration displayed a trend towards a thicker AoIMT vs. the AC allele. Analysis of the AA, AC, and CC allele groups showed significant differences in the ejection fraction (AA vs. AC, 50% vs. 59%; P = 0.043; data not shown). There were no significant differences in any of the other variables between the groups.
| Genotype | All AoIMT (mm) | BAV AoIMT (mm) | TAV AoIMT (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele | ||||||||||
| ANOVA | n | ANOVA | n | ANOVA | n | |||||
| rs488378 | AA | ns | 52 | 1.38 ± 0.30 | ns | 28 | 1.33 ± 0.28 | ns | 24 | 1.42 ± 0.32 |
| AG | 163 | 1.39 ± 0.31 | 93 | 1.33 ± 0.35 | 70 | 1.45 ± 0.25 | ||||
| GG | 122 | 1.36 ± 0.36 | 70 | 1.28 ± 0.32 | 52 | 1.45 ± 0.39 | ||||
| rs200991 | AA | 0.063 | 5 | 1.54 ± 0.27 | ns | 3 | 1.45 ± 0.13 | 0.042‡ | 2 | 1.68 ± 0.44 |
| AC | 89 | 1.31 ± 0.29 | 56 | 1.30 ± 0.32 | 33 | 1.33 ± 0.26 | ||||
| CC | 243 | 1.40 ± 0.34 | 132 | 1.32 ± 0.34 | 111 | 1.48 ± 0.32 | ||||
AoIMT, aortic intima-media thickness; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; n, number of patients; ANOVA, analysis of variance. Tukeys test for post hoc analysis between groups, ‡CC vs. AC (P = 0.056). For further analysis, we divided the patients into two groups: A allele (AA and AC) vs. non A allele (CC) in the rs200991 locus.
3.2. Correlations
There were significant correlations (P < 0.05) between AoIMT and age (r = 0.48) and hsCRP (r = 0.11). Descending aortic dimension was age-dependent (r = 0.62, P < 0.001)).
For further analysis, we divided the patients into two groups: A allele (shaded areas in Table 3) vs. non A allele in the rs200991 locus. The results of the univariate regression analysis for AoIMT are shown in Table 4, there were no associations between height, hypertension, cholesterol, LDL or ascending aortic dimension adjusted for BSA and AoIMT for the whole group or BAV/TAV separately. In our multivariate model, we chose variables of clinical relevance or variables shown to be associated with CIMT or AoIMT in previous studies, such as smoking and hsCRP. As a marker of AS, we choose only Pmax because there is strong correlations between all the other AS variables. For AR, we included only the AR grade in the model. We used the MBP to represent the SBP and DBP. Gender is a known determinant of CIMT and was included in the multivariate model, despite it not being significant in the univariate analysis. The multivariate analysis for the whole group showed that AoIMT was associated with age and male gender (Table 5). When the BAV and TAV subgroups were analyzed separately, age was still the main predictor of AoIMT in both groups. The rs200991 SNP genotype was significant (P = 0.034) for the TAV group of patients, and the creatinine level was significant for the BAV group (P = 0.019). Smoking, Pmax and hsCRP, were not associated with AoIMT in the multivariate analysis.
| All | BAV | TAV | ||||
|---|---|---|---|---|---|---|
| βeta | P | βeta | P | βeta | P | |
| Age | 0.482 | < 0.001 | 0.474 | < 0.001 | 0.402 | < 0.001 |
| Male gender | − 0.006 | 0.910 | − 0.028 | 0.689 | 0.070 | 0.384 |
| Inclusion SBP | 0.080 | 0.126 | 0.150 | 0.030 | − 0.080 | 0.322 |
| Inclusion DBP | 0.155 | 0.003 | 0.197 | 0.004 | 0.205 | 0.010 |
| Mean BP | 0.151 | 0.004 | 0.214 | 0.002 | 0.101 | 0.208 |
| Ever smoker | 0.134 | 0.011 | 0.170 | 0.015 | 0.124 | 0.123 |
| Creatinine | 0.115 | 0.029 | 0.126 | 0.071 | 0.065 | 0.419 |
| lnhsCRP | 0.143 | 0.006 | 0.092 | 0.187 | 0.139 | 0.086 |
| lnTrigycerides | 0.036 | 0.488 | 0.141 | 0.041 | − 0.084 | 0.300 |
| HDL | 0.117 | 0.026 | 0.229 | 0.001 | − 0.011 | 0.896 |
| Pmax | 0.173 | 0.001 | 0.151 | 0.032 | 0.289 | < 0.001 |
| AR (0–3) | − 0.248 | < 0.001 | − 0.251 | < 0.001 | − 0.289 | < 0.001 |
| AVA | − 0.248 | < 0.001 | − 0.240 | 0.001 | − 0.328 | < 0.001 |
| AoDesc | 0.352 | < 0.001 | 0.347 | < 0.001 | 0.290 | 0.001 |
| AoDesc/BSA | 0.329 | < 0.001 | 0.316 | < 0.001 | 0.278 | 0.002 |
| BAV | − 0.209 | < 0.001 | – | – | – | – |
| rs200991A | − 0.098 | 0.073 | − 0.028 | 0.699 | − 0.168 | 0.043 |
AoDsc, descending aortic diameter; AoIMT, aortic intima-media thickness; AR, aortic regurgitation; AVA, aortic valve area; BAV, bicuspid aortic valve; BSA, body surface area; DBP, diastolic blood pressure; HDL, high-density lipoprotein; EF, ejection fraction; Mean BP, mean blood pressure; LVEDD, left ventricular end-diastolic diameter; Pmax, aortic valve peak gradient; SBP, systolic blood pressure; TAV, tricuspid aortic valve; lnhsCRP, natural logarithm of highly sensitive C-reactive protein level; lnTrigycerides, natural logarithm of triglyceride levels; rs200991A, A allele rs200991.
| All (R2 = 0.241) | BAV (R2 = 0.252) | TAV (R2 = 0.181) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| βeta | P | R2 change | βeta | P | R2 change | βeta | P | R2 change | |
| Age (years) | 0.467 | < 0.001 | 0.214 | 0.419 | < 0.001 | 0.217 | 0.337 | < 0.001 | 0.248 |
| Male gender | 0.121 | 0.020 | 0.012 | ||||||
| Creatinine (μml/L) | 0.159 | 0.019 | 0.024 | ||||||
| HDL | 0.119 | 0.087 | 0.013 | ||||||
| AR (grades 0–3) | − 0.094 | 0.079 | 0.008 | − 0.044 | 0.069 | 0.020 | |||
| rs200991A | − 0.095 | 0.059 | 0.008 | − 0.166 | 0.034 | 0.027 | |||
| Mean BP (mm Hg) | 0.119 | 0.082 | 0.013 | ||||||
| BAV | |||||||||
AoIMT, aorta intima-media thickness; AR, aortic regurgitation; BAV, bicuspid aortic valve; BSA, body surface area; HDL, high-density lipoprotein; TAV, tricuspid aortic valve; rs200991A, A allele of the rs200991 SNP; Mean BP, mean blood pressure.
4. Discussion
Descending aortic IMT in patients with aortic valve stenosis or regurgitation and/or AscAA, but without significant CAD, is mainly determined by age and gender, and is not influenced by the presence of a BAV, known to be associated with aortopathy. In patients with a TAV, we found IMT in the descending aorta to be associated with the rs200991 SNP genotype in the HIST1H2BN locus, recently identified as a genetic determinant of CIMT.
To the best of our knowledge, this is the first study examining IMT in the descending aorta with a focus on possible associations with aortic valve phenotype and aortic valve disease. We were especially interested to explore whether BAV morphology, known to be associated with aortopathy [1]; [23] ; [24] and abnormal, strong helical flow pattern in the entire aorta [8], has an impact on AoIMT. In our study of 368 patients we found no correlations between AoIMT and valve phenotype, variables of AS or AR and in multivariate analysis. The difference in AoIMT seen between the BAV and TAV groups could be explained mainly by a difference in age. Although impairments of elastic properties of the descending aorta have been identified in patients with a BAV [9] ; [11], it seems not associated with dilatation or altered AoIMT in this aortic segment as shown in our study and by others [10]. Although the main focus of this study was the descending aorta, it can be mentioned that histological analysis of the ascending aorta in patients with BAV and AscAA have shown less thinning of IMT [25] and fewer intimal changes [26] compared with patients with a TAV and AscAA. Our group has previously examined the genetic, molecular and biomechanical properties of the ascending aorta in patients with AscAA in the ASAP patient population and found significant differences between BAV and TAV. A combined proteomic and transcriptomic approach showed that AscAA formation in patients with BAV and TAV involves different biological pathways leading to the same phenotype [27]. Also BAV patients have an impaired mechanism for the splicing of extra domain A of fibronectin, supporting that dilatation of the ascending aorta relates to an inherent tissue weakness in BAV patients [28]. The examination of sections of aneurysmal aortic tissue in BAV patients showed a higher macroscopic strength, caused by an increased collagen-related stiffness, but the elastin-related stiffness was similar in BAV and TAV AscAAs [1]. The function of the ascending aorta in vivo has been extensively examined by others [2]; [3] ; [4].
It is debated whether BAV aortopathy is caused by a genetic predisposition and/or the result from altered blood flow with proponents of both theories. A combination of genetic, flow and environmental factors in the individual patient is probably responsible for AscAA formation [29]. The fact that microanatomy in the descending aorta (AoIMT) did not differ between BAV and TAV does not exclude differences in aortic function, or inherent tissue weakness as the etiology of BAV aortopathy [30].
IMT in the aorta has been studied mainly in the abdominal segment. An increase of IMT in this vessel with age can be seen even in neonates of different gestational ages [31]. In our study male gender was a significant positive determinant of AoIMT, even after correcting for age, as the male subjects were younger than the female ones (62 ± 13 vs. 69 ± 10 years). We found no correlations with known atherosclerotic risk factors such as cholesterol, LDL, HDL, or triglycerides, probably because we selected low-risk patients lacking signs of CAD, and with lipid levels generally within normal limits. Although there are known to be associations between the hsCRP concentration and aortic stiffness [32], we did not find the hsCRP level to be a significant predictor of AoIMT after correcting for age in our patients. As far as we know, an association between IMT in the descending aorta and hsCRP levels has not been studied yet.
After adjusting for age and traditional risk factors, the rs200991 SNP became a significant determinant of AoIMT only among patients with a TAV. Gertow et al. reported a positive association for the A allele on CIMT values among patients with known multiple cardiovascular risk factors [15], whereas we found a negative association in patients with aortic valve disease, but otherwise low risk of atherosclerosis, without CAD and lipids levels close to normal limits. Needless to say, larger studies are required to obtain firm conclusions in different patient populations.
The methodology of measuring IMT in the aorta has varied in different studies and different IMT variables have been reported, namely the maximal and/or mean IMT. In most studies, single calipers applied to B- or M-mode images have been used [32] ; [33]. There are few publications on measuring the aortic IMT using semi-automated edge-detection methods in a 10-mm-wide vessel segment, reporting mean IMT value [34].
Although a long-axis image of vessels is a standard projection for measurements of IMT, however, there are reports on AoIMT measurements in the transverse plane, which has been used in this study [35] ; [36]. The descending aorta has a diameter of approximately 21–26 mm with a large curvature allowing IMT measurement over a 10-mm sector on the short axis image. CT and MRI have the advantage of imaging the whole aorta as well as the entire circumference of the vessel. However the spatial resolution is considerably lower [37] ; [38], making echocardiography a method of choice for IMT measurements.
5. Limitations
Using an anatomical landmark, such as the left atrium instead of a fixed distance from the teeth, would have compensated for patient height and ensured that AoIMT was analyzed in the same region in all patients. Due to multiple testing there is always a risk of type B error, which we tried to avoid by limiting the number of variables tested keeping the ratio between number of patients and independent variables at < 10 also in the BAV group.
6. Conclusion
Intima-media thickness of the descending aorta is not directly affected by aortic valve morphology (BAV/TAV); age is the main determinant of AoIMT. Our results regarding AoIMT do not exclude differences in aortic function, or inherent tissue weakness as the etiology to BAV aortopathy. Our study also identified a genetic determinant of AoIMT in patients with a TAV, while in patients with a BAV the AoIMT was associated with creatinine levels.
Conflict of interest
Relationship with industry: None. The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
We would like to thank Elisabeth Berg MSc, LIME/MedStat, Karolinska Institutet, for the valuable statistical support. The study was supported by the Swedish Research Council (diary number 2011-3579; K2012-65X-22036-01-3), the Swedish Heart Lung Foundation (project number 20120209), the Stockholm County Council (grant number 20120628, 20130301), and a donation by Fredrik Lundberg.
References
- [1] C. Forsell, H.M. Bjorck, P. Eriksson, A. Franco-Cereceda, T.C. Gasser; Biomechanical properties of the thoracic aneurysmal wall: differences between bicuspid aortic valve and tricuspid aortic valve patients; Ann. Thorac. Surg., 98 (2014), pp. 65–71
- [2] I. Oulego-Erroz, P. Alonso-Quintela, M. Mora-Matilla, S. Gautreaux Minaya, S. Lapena-Lopez de Armentia; Ascending aorta elasticity in children with isolated bicuspid aortic valve; Int. J. Cardiol., 168 (2013), pp. 1143–1146
- [3] B.M. Schaefer, M.B. Lewin, K.K. Stout, P.H. Byers, C.M. Otto; Usefulness of bicuspid aortic valve phenotype to predict elastic properties of the ascending aorta; Am. J. Cardiol., 99 (2007), pp. 686–690
- [4] C.Y. Shim, I.J. Cho, W.I. Yang, M.K. Kang, S. Park, J.W. Ha, et al.; Central aortic stiffness and its association with ascending aorta dilation in subjects with a bicuspid aortic valve; J. Am. Soc. Echocardiogr., 24 (2011), pp. 847–852
- [5] S.C. Siu, C.K. Silversides; Bicuspid aortic valve disease; J. Am. Coll. Cardiol., 55 (2010), pp. 2789–2800
- [6] H.I. Michelena, A.D. Khanna, D. Mahoney, E. Margaryan, Y. Topilsky, R.M. Suri, et al.; Incidence of aortic complications in patients with bicuspid aortic valves; JAMA, 306 (2011), pp. 1104–1112
- [7] E. Girdauskas, M. Rouman, K. Disha, T. Scholle, B. Fey, B. Theis, et al.; Correlation between systolic transvalvular flow and proximal aortic wall changes in bicuspid aortic valve stenosis; Eur. J. Cardiothorac. Surg. (2014)
- [8] R. Lorenz, J. Bock, A.J. Barker, F. von Knobelsdorff-Brenkenhoff, W. Wallis, J.G. Korvink, et al.; 4D flow magnetic resonance imaging in bicuspid aortic valve disease demonstrates altered distribution of aortic blood flow helicity; Magn. Reson. Med. (2013)
- [9] H.B. Grotenhuis, J. Ottenkamp, J.J. Westenberg, J.J. Bax, L.J. Kroft, A. de Roos; Reduced aortic elasticity and dilatation are associated with aortic regurgitation and left ventricular hypertrophy in nonstenotic bicuspid aortic valve patients; J. Am. Coll. Cardiol., 49 (2007), pp. 1660–1665
- [10] L.J. Martin, R.B. Hinton, X. Zhang, L.H. Cripe, D.W. Benson; Aorta measurements are heritable and influenced by bicuspid aortic valve; Front. Genet., 2 (2011), p. 61
- [11] J. Petrini, J. Jenner, A. Rickenlund, P. Eriksson, A. Franco-Cereceda, K. Caidahl, et al.; Elastic properties of the descending aorta in patients with a bicuspid or tricuspid aortic valve and aortic valvular disease; J. Am. Soc. Echocardiogr., 27 (2014), pp. 393–404
- [12] J.H. Bae, E. Bassenge, K.R. Park, K.Y. Kim, M. Schwemmer; Significance of the intima-media thickness of the thoracic aorta in patients with coronary atherosclerosis; Clin. Cardiol., 26 (2003), pp. 574–578
- [13] J.J. Chiu, S. Chien; Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives; Physiol. Rev., 91 (2011), pp. 327–387
- [14] C. Wang, B.M. Baker, C.S. Chen, M.A. Schwartz; Endothelial cell sensing of flow direction; Arterioscler. Thromb. Vasc. Biol., 33 (2013), pp. 2130–2136
- [15] K. Gertow, B. Sennblad, R.J. Strawbridge, J. Ohrvik, D. Zabaneh, S. Shah, et al.; Identification of the BCAR1-CFDP1-TMEM170A locus as a determinant of carotid intima-media thickness and coronary artery disease risk; Circ. Cardiovasc. Genet., 5 (2012), pp. 656–665
- [16] H. Baumgartner, J. Hung, J. Bermejo, J.B. Chambers, A. Evangelista, B.P. Griffin, et al.; Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice; J. Am. Soc. Echocardiogr., 22 (2009), pp. 1–23 (quiz 101-102)
- [17] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J. Am. Soc. Echocardiogr., 18 (2005), pp. 1440–1463
- [18] W.A. Zoghbi, M. Enriquez-Sarano, E. Foster, P.A. Grayburn, C.D. Kraft, R.A. Levine, et al.; Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography; J. Am. Soc. Echocardiogr., 16 (2003), pp. 777–802
- [19] V. Jackson, M.J. Eriksson, K. Caidahl, P. Eriksson, A. Franco-Cereceda; Ascending aortic dilatation is rarely associated with coronary artery disease regardless of aortic valve morphology; J. Thorac. Cardiovasc. Surg. (2014)
- [20] H.H. Sievers, C. Schmidtke; A classification system for the bicuspid aortic valve from 304 surgical specimens; J. Thorac. Cardiovasc. Surg., 133 (2007), pp. 1226–1233
- [21] L. Folkersen, F. van't Hooft, E. Chernogubova, H.E. Agardh, G.K. Hansson, U. Hedin, et al.; Association of genetic risk variants with expression of proximal genes identifies novel susceptibility genes for cardiovascular disease; Circ. Cardiovasc. Genet., 3 (2010), pp. 365–373
- [22] S. Rodriguez, T.R. Gaunt, I.N. Day; Hardy–Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies; Am. J. Epidemiol., 169 (2009), pp. 505–514
- [23] L. Folkersen, D. Wagsater, V. Paloschi, V. Jackson, J. Petrini, S. Kurtovic, et al.; Unraveling divergent gene expression profiles in bicuspid and tricuspid aortic valve patients with thoracic aortic dilatation: the ASAP study; Mol. Med., 17 (2011), pp. 1365–1373
- [24] V. Jackson, T. Olsson, S. Kurtovic, L. Folkersen, V. Paloschi, D. Wagsater, et al.; Matrix metalloproteinase 14 and 19 expression is associated with thoracic aortic aneurysms; J. Thorac. Cardiovasc. Surg., 144 (2012), pp. 459–466
- [25] A. Forte, A. Della Corte, M. Grossi, C. Bancone, R. Provenzano, M. Finicelli, et al.; Early cell changes and TGFbeta pathway alterations in the aortopathy associated with bicuspid aortic valve stenosis; Clin. Sci. (Lond.), 124 (2013), pp. 97–108
- [26] M.J. Collins, V. Dev, B.H. Strauss, P.W. Fedak, J. Butany; Variation in the histopathological features of patients with ascending aortic aneurysms: a study of 111 surgically excised cases; J. Clin. Pathol., 61 (2008), pp. 519–523
- [27] S. Kjellqvist, S. Maleki, T. Olsson, M. Chwastyniak, R.M. Branca, J. Lehtio, et al.; A combined proteomic and transcriptomic approach shows diverging molecular mechanisms in thoracic aortic aneurysm development in patients with tricuspid- and bicuspid aortic valve; Mol. Cell. Proteomics, 12 (2013), pp. 407–425
- [28] V. Paloschi, S. Kurtovic, L. Folkersen, D. Gomez, D. Wagsater, J. Roy, et al.; Impaired splicing of fibronectin is associated with thoracic aortic aneurysm formation in patients with bicuspid aortic valve; Arterioscler. Thromb. Vasc. Biol., 31 (2011), pp. 691–697
- [29] N. Abdulkareem, J. Smelt, M. Jahangiri; Bicuspid aortic valve aortopathy: genetics, pathophysiology and medical therapy; Interact. Cardiovasc. Thorac. Surg. (2013)
- [30] G. Pepe, R. De Cario, E. Sticchi, B. Giusti, R. Abbate, G. Gensini, et al.; Bicuspid aortic valve syndrome and fibrillinopathies: potential impact on clinical approach; 1 (2015) (2015), p. 8
- [31] E. Koklu, S. Kurtoglu, M. Akcakus, A. Yikilmaz, A. Coskun, T. Gunes; Intima-media thickness of the abdominal aorta of neonate with different gestational ages; J. Clin. Ultrasound, 35 (2007), pp. 491–497
- [32] G. Qureshi, R. Brown, L. Salciccioli, M. Qureshi, S. Rizvi, S. Farhan, et al.; Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness; Atherosclerosis, 195 (2007), pp. e190–e194
- [33] G. Couturier, A. Voustaniouk, J. Weinberger, V. Fuster; Correlation between coronary artery disease and aortic arch plaque thickness measured by non-invasive B-mode ultrasonography; Atherosclerosis, 185 (2006), pp. 159–164
- [34] E.M. Dahlen, T. Andreasson, M. Cinthio, F.H. Nystrom, C.J. Ostgren, T. Lanne; Is there an underestimation of intima-media thickness based on M-mode ultrasound technique in the abdominal aorta?; Clin. Physiol. Funct. Imaging, 32 (2012), pp. 1–4
- [35] A. Nemes, T. Forster, M.L. Geleijnse, O.I. Soliman, F.J. Ten Cate, M. Csanady; Prognostic value of coronary flow reserve and aortic distensibility indices in patients with suspected coronary artery disease; Heart Vessels, 23 (2008), pp. 167–173
- [36] C.A. Roldan, J. Joson, C.R. Qualls, J. Sharrar, W.L. Sibbitt Jr.; Premature aortic stiffness in systemic lupus erythematosus by transesophageal echocardiography; Lupus, 19 (2010), pp. 1599–1605
- [37] B. Mensel, A. Quadrat, T. Schneider, J.P. Kuhn, M. Dorr, H. Volzke, et al.; MRI-based determination of reference values of thoracic aortic wall thickness in a general population; Eur. Radiol. (2014)
- [38] F. Rengier, P. Geisbusch, R. Vosshenrich, M. Muller-Eschner, C. Karmonik, P. Schoenhagen, et al.; State-of-the-art aortic imaging: part I — fundamentals and perspectives of CT and MRI; VASA Z. Gefasskrankheiten, 42 (2013), pp. 395–412
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?