(Created page with "==Summary== ====Background and aim==== Barretts esophagus (BE) is the most common cause or precursor of esophageal adenocarcinoma (EAC), a condition with a poor prognosis. T...") |
m (Scipediacontent moved page Draft Content 836782505 to Wang et al 2015g) |
(No difference)
| |
Latest revision as of 12:01, 15 May 2017
Summary
Background and aim
Barretts esophagus (BE) is the most common cause or precursor of esophageal adenocarcinoma (EAC), a condition with a poor prognosis. This study aimed to investigate the clinical characteristics and risk of EAC in patients with BE.
Materials and methods
From January 2001 to December 2012, a total of 425 patients with histologically proven BE were identified and analyzed retrospectively. Patients' personal data (smoking, alcohol consumption), underlying systemic disease data (diabetes mellitus and hypertension), endoscopic findings (hiatal hernia, peptic ulcer, endoscopically suspected esophageal metaplasia, severity of reflux esophagitis, rapid urease test), and pathological findings (degree of dysplasia, Helicobacter pylori infection) were collected for further analysis.
Results
In this retrospective study, 15 patients were diagnosed with EAC. Only one patient was found to have EAC during endoscopic surveillance for BE. The majority of patients (14/15 patients) suffered alarm symptoms and were diagnosed to have BE and EAC concurrently. Meanwhile, EAC is already relatively at an advanced stage. The mean age for diagnosis of EAC in a patient with BE was 67.67 ± 9.92 years old. All patients were male. From a total of 15 patients, 33.3% (5 patients) demonstrated erosive esophagitis under endoscopy and 60% (3 of 5 patients) of these were classified as Los Angeles grade C or D disease.
Conclusion
Our study found that BE-associated EAC mostly occurred in older men. In the group with BE-associated EAC, the majority of patients were discovered due to alarm symptoms, at the same time as esophageal adenocarcinoma had already developed. Further prospective study is needed to stratify the risk of disease progression in BE patients.
Keywords
Barretts esophagus ; Esophageal adenocarcinoma ; Reflux esophagitis
Introduction
Barretts esophagus (BE) is the most common cause or precursor of esophageal adenocarcinoma (EAC) and has a poor prognosis. The overall risk of cancer developing in BE patients is estimated to be approximately 0.12–0.5% per year [1] ; [2] ; [3] ; [4] . The prognosis is linked strongly to the stage of the tumor at the time of detection. The 5-year survival rate can be as high as 83–90% in the early stages, compared with only 10–15% in late-stage cancers [5] ; [6] ; [7] . Chronic esophageal acid exposure, being male, old age, visceral obesity, hiatus hernia, smoking, and alcohol consumption are risk factors for BE in Asia. Helicobacter pylori infection showed a protective effect on BE [8] . In Western countries, BE has been diagnosed in approximately 10–15% of patients undergoing an endoscopy for gastroesophageal reflux disease (GERD) symptoms [1] . Although GERD is less prevalent in Asia than in the West, its incidence is rapidly rising in Asia due to the change to Western-style eating habits [9] . EAC has increased progressively with an increased prevalence of GERD in Western countries; whereas in Asia, despite the increased prevalence of GERD, EAC has not increased accordingly [8] ; [10] . However, esophageal squamous cell carcinoma is still the dominant cell type of esophageal cancer in Asian countries. Our retrospective study aimed to investigate the clinical characteristics and risk of EAC in patients with BE in Taiwan.
Materials and methods
Patient selection
A total of 425 patients, first diagnosed with BE histopathologically between 2001 and 2012, were identified retrospectively by reviewing pathological reports at Chang Gung Memorial Hospital, Linkou, Taiwan. These patients were analyzed using personal characteristics, including age, sex, smoking and alcohol usage, underlying systemic disease (diabetes mellitus and hypertension), and indications from endoscopic examination; endoscopic findings (hiatal hernia, peptic ulcers, severity of reflux esophagitis, rapid urease test, and endoscopically suspected esophageal metaplasia); and, pathological findings (degree of dysplasia, H. pylori infection). Rapid urease test was used to determine the presence of H pylori and it depended on clinical indication. However, not all BE patients received the rapid urease test in this retrospective study. The patients were then analyzed using the above information to identify the risks for EAC in the Barretts population. This study was carried out with preapproval by the Linkou Chang-Gung Memorial Hospital Institutional Review Board (102-2699B).
Endoscopy and definition of BE
Indications of the endoscopies were collected from the descriptions on the endoscopic official reports and comprised the typical reflux symptoms (including acid regurgitation and heart burn), abdominal pain, asymptomatic or surveillance of BE, gastrointestinal (GI) tract bleeding, chest pain, dysphagia, and other causes. Reflux esophagitis was diagnosed using endoscopy and classified into grades A–D using the Los Angeles (LA) classification [11] ; [12] . Endoscopically suspected esophageal metaplasia (ESEM) was defined as a length of columnar epithelium that extended continuously from the gastric lumen to the esophagus and was recorded using Prague Barretts C(circumferential) & M(maximal extent) criteria [13] . The length of BE was not measured using Prague Barretts C & M criteria prior to 2006. The definition of BE using pathological criteria requires that intestinal metaplasia with goblet cells is present in the esophageal columnar-lined mucosa of the esophagus.
Statistical analysis
We performed statistical analyses on the acquired data using SPSS version 20.0(Armonk, NY: IBM Corp). Nine variables, including sex, age, indication of esophagogastroduodenoscopy (EGD), smoking history, alcohol use, hiatal hernia, the presence of erosive esophagitis, and underlying diseases (diabetes and hypertension) were considered as potential risk factors for adenocarcinoma among the BE patients. Quantitative data were reported as means and standard deviation, whereas categorical data were expressed as proportions.
The data were analyzed using the Student t test for continuous variables, and Chi-square test for categorical variables.
Univariate analysis was performed to assess the effect of each variable. Logistic regression was used to identify individual correlations associated with adenocarcinoma risk. Using the backward stepwise method, variables with a p value < 0.05 were included in the multivariate logistic regression models. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated where appropriate. A two-sided p value < 0.05 was considered to be significant in all statistical tests.
Results
Barretts population
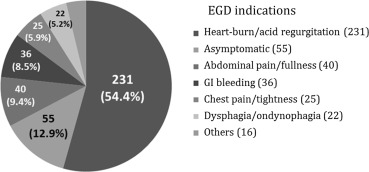
In the total study population, 313 patients (73.6%) were males with a mean age of 57.04 ± 17.38 years, and the male-to-female ratio was 2.8:1. Adenocarcinoma was diagnosed in 15 patients, 100% of whom were male and had a mean age of 67.67 ± 9.92 years. Patient characteristics are shown in Table 1 . Indications for endoscopic examination (Fig. 1 ) included typical reflux symptoms (acid regurgitation and heart burn) in 231 (54.4%) patients, asymptomatic or endoscopy followed up in 55 (12.9%) patients, abdominal pain in 40 (9.4%) patients, GI tract bleeding in 36 (8.5%) patients, dysphagia in 22 (5.2%) patients, chest pain in 25 (5.9%) patients, and other causes in 16 (3.8%) patients.
| Total (n = 425) | BE (n = 410) | BE with adenocarcinoma (n = 15) | p | |
|---|---|---|---|---|
| Male (%) | 313 (73.6) | 298 (72.7) | 15 (100) | 0.018 |
| Age (y), mean ± SD | 57.04 ± 17.38 | 56.65 ± 17.49 | 67.67 ± 9.92 | 0.009 |
| EGD indication | ||||
| Heartburn/regurgitation (%) | 231 (54.4) | 225 (54.9) | 6 (40) | 0.256 |
| Asymptomatic (%) | 55 (12.9) | 55 (13.4) | 0 (0) | 0.128 |
| Abdominal pain (%) | 40 (9.4) | 40 (9.8) | 0 (0) | 0.204 |
| GI bleeding (%) | 36 (8.5) | 33 (8.0) | 3 (20) | 0.103 |
| Chest pain (%) | 25 (5.9) | 25 (6.1) | 0 (0) | 0.324 |
| Dysphagia (%) | 22 (5.2) | 16 (3.9) | 6 (40) | <0.001 |
| Reflux esophagitis | ||||
| Yes (%) | 271 (63.8) | 266 (64.9) | 5 (33.3) | 0.013 |
| Grade A (%) | 133 (31.3) | 132 (32.2) | 3 (20) | |
| Grade B (%) | 83 (19.5) | 82 (20) | 1 (6.7) | |
| Grade C (%) | 26 (6.1) | 26 (6.3) | 0 (0) | |
| Grade D (%) | 29 (6.8) | 26 (6.3) | 1 (6.7) | |
| Hiatal hernia (%) | 124 (29.2) | 121 (29.5) | 3 (20) | 0.426 |
| Helicobacter pylori (%) | 49/94 (52.1) | 48/93 (51.6) | 1/1 (100) | |
| Smoking (%) | 144 (33.9) | 139 (33.9) | 5 (33.3) | 0.964 |
| Alcohol (%) | 84 (19.8) | 78 (19.0) | 6 (40) | 0.045 |
| Diabetes mellitus (%) | 70 (16.5) | 67 (16.3) | 3 (20) | 0.707 |
| Hypertension (%) | 132 (31.1) | 128 (31.5) | 4 (26.7) | 0.708 |
- p value is significant using Chi-square test.
BE = Barretts esophagus; EGD = esophagogastroduodenoscopy; GI = gastrointestinal; SD = standard deviation.
|
|
|
Figure 1. Esophagogastroduodenoscopy ( EGD) indications in the study patients (n = 425). |
Endoscopic findings
Among the 425 patients with BE, 271 (63.8%) patients were diagnosed with reflux esophagitis, and according to the LA classification system 133 (31.3%) patients were classified as grade A, 83 (19.5%) patients as grade B, 26 (6.1%) patients as grade C, and 29 (6.8%) patients as grade D. There were 49 of 94 patients (52.1%) who recorded a positive finding on the H. pylori test and 124 of 425 patients (29.2%) who had a hiatal hernia. We analyzed the endoscopic findings of BE cases according to the Prague criteria which was published on 2006. The mean length of the circumferential extent was 2.08 cm (range: 0–9 cm) and the mean length of the maximal extent was 3.67 cm (range: 1–10 cm). There are a total of 83 BE cases classified by Prague C & M criteria in our study group. According to the C & M criteria, 43 cases can be classified as short segment and 40 cases as long segment.
Pathological findings
In the initial biopsy specimens, nine patients (2.1%) were found to have dysplastic mucosa, eight patients had low-grade dysplasia, and one patient had high-grade dysplasia. A total of 15 patients (3.5%) were diagnosed with adenocarcinoma in our study group, including one patient with high-grade dysplasia initially, but patient was lost to follow up. Esophageal adenocarcinoma developed 31 months later.
The mean number of times and the duration of endoscopic surveillance for the low-grade dysplasia of BE were 1.9 times (1.9 ± 1.1) and 9 months (9 ± 9.3) without progression, respectively.
EAC in the BE population
A total of 15 patients with BE and EAC concurrently were recorded in our study (Table 1 ). They were all male patients with a mean age of 67.67 ± 9.92 years. The majority (14/15 patients) were diagnosed with EAC at the first time of EGD. One patient was under regular surveillance for BE with a previous finding of reflux esophagitis LA grade D and high-grade dysplasia. Indications for endoscopic examination revealed dysphagia (6/15 patients, 40%), heart burn or acid regurgitation (6/15 patients, 40%), and GI bleeding (3/15 patients, 20%). Endoscopic findings reported reflux esophagitis (5/15 patients, 33.3%) including LA grade A and B (4/15 patients, 26.7%) and LA grade C and D (1/15 patients, 6.7%). Hiatal hernia was noted in 20% of these patients (3/15). Eight people received surgical resection and esophageal reconstruction, depending on whether or not the lesions were involved with the cardia; two patients received endoscopic mucosal resection; two patients received concurrent chemo-radiotherapy; and three patients were lost to follow up.
In the Chi-square test analysis model (Table 1 ), male sex (p = 0.018), dysphagia as an indication of EGD (p < 0.001), reflux esophagitis (p = 0.013), and alcohol consumption (p = 0.045), and in the Student t test model age at diagnosis of BE (p = 0.009) were identified as potential risk factors for BE. An age of ≥ 57 years at diagnosis of BE was associated with an AUROC (Area Under the Receiver Operating Characteristic) of 0.70 (95% CI: 0.606–0.788, p = 0.009). In the univariate analysis model, age at diagnosis of BE (≥ 57 years; OR = 14.4, 95% CI = 1.88–110.6, p = 0.01), dysphagia as an EGD indication (OR = 16.4, 95% CI = 5.21–51.7, p = 0.001), and reflux esophagitis (OR = 0.27, 95% CI = 0.09–0.81, p = 0.019) were risk factors for the development of EAC ( Table 2 ). In the multivariate analysis model, age at diagnosis of BE (≥ 57 years; OR = 33.9, 95% CI = 3.54–324.3, p = 0.002) and dysphagia as an EGD indication (OR = 43.5, 95% CI = 9.56–198.4, p < 0.001) were independent risk factors for the development of EAC ( Table 2 ).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds Ratio (95%) | p | Odds Ratio (95%) | p | |
| Age > 57 y | 14.4 (1.88–110.6) | 0.010 | 33.9 (3.54–324.3) | 0.002 |
| Dysphagia | 16.4 (5.21–51.7) | 0.001 | 43.5 (9.56–198.4) | < 0.001 |
| Reflux esophagitis | 0.27 (0.09–0.81) | 0.019 | ||
| Alcohol | 2.84 (0.98–8.21) | 0.054 | ||
Discussion
This retrospective study analyzed patients with histologically confirmed BE and revealed that being male, old age, and alarm symptoms (especially dysphagia) are the clinical characteristics of a BE patient with EAC.
The epidemiology data of BE prevalence in Taiwan are limited and diverse. Lee et als [14] analysis of 4600 consecutive health check-up participants revealed a prevalence of 0.3%. In another study among 19,812 participants receiving an endoscopy during a health examination, the prevalence of ESEM and histologically confirmed BE were 0.28% and 0.06%, respectively [15] . In a previous study, 13 BE cases were found in 736 consecutive patients undergoing endoscopy for a variety of GI symptoms, with a prevalence of 1.8% [16] , similar to the findings reported by Yeh et al [17] . Based on the standardized protocol with random four-quadrant endoscopic biopsy, Chang et al [18] revealed that the prevalence of BE among patients who had a referral endoscopy, screening endoscopy, and overall was 1.06%, 0.35%, and 0.85%, respectively.
Sex differences have been observed in some esophageal diseases and the reasons for such differences still require further study. The incidence rate of GERD is similar between men and women [19] . However, the incidence rate of BE, EAC, or BE-associated EAC is higher in men than in women [2] ; [20] ; [21] ; [22] ; [23] . One meta-analysis of cohort studies showed that the risk of BE among men is two-fold higher than for women [23] . Edgren et al [20] reported that being male was a risk factor for esophageal and junctional adenocarcinoma by seven-fold. One nationwide cohort study showed that high-grade dysplasia and EAC in the BE population have a 2:1 male predominance (OR: 2.01; 95% CI = 1.68–2.60) [2] .
Several investigations have identified the clinical features and characteristics of patients with esophageal adenocarcinoma, including being male, the presence of BE, gastroesophageal reflux, central obesity [24] , decreasing prevalence of H. pylori infection, hiatal hernia, and low fruit and vegetable consumption [2] ; [4] ; [22] ; [25] ; [26] . One meta-analysis study revealed the symptoms of GERD increased the odds of developing EAC by five-fold to seven-fold [21] . Although patients with BE have a high relative risk of developing adenocarcinoma of the esophagus (30–125 times higher risk than the general population), their absolute risk is low. The risk of developing EAC for patients with BE is approximately 0.12–0.5% per year [4] . It is important to identify the clinical characteristics of patients who have BE-associated EAC and to commence endoscopic surveillance for patients with a high risk of BE-associated EAC (such as male sex) before obvious alarm symptoms occur.
In our study, 54.4% of the patients diagnosed with BE underwent EGD due to typical reflux symptoms. The severity of reflux esophagitis in most of these people was classified as L A grade A or B. As in previous reports, there was a strong association between chronic symptomatic gastroesophageal reflux and BE [22] ; [27] ; [28] ; [29] . The reflux of gastroduodenal contents into the esophagus is considered to be a risk factor for carcinogenesis due to chronic esophageal injury.
In our endoscopic findings for BE-associated EAC, polypoid lesions were found in 86.7% (13/15) of the patients compared to ulcerative lesions in only 13.3% of patients. Dysphagia, gastroesophageal bleeding, or body weight loss were common presentations of BE-associated adenocarcinoma in this population. Our findings are in agreement with the report by Gatenby et al [30] . It was concluded that there were strong associations between the symptoms of dysphagia/odynophagia/weight loss and the development of high-grade dysplasia and EAC [30] . A similar study also reported that obvious lumps or nodules located endoscopically suggest that a more advanced lesion with invasion may be present [31] ; [32] ; [33] .
Our observations also discovered that all BE-associated adenocarcinoma were men with an older mean age than the BE population. Male sex and older age were identified as important independent risk factors for progression to high-grade dysplasia and EAC [2] ; [21] ; [34] .
There are some limitations to the present study. It is possible that the number of BE patients has been underestimated because endoscopically suspected esophageal metaplasia may not have been routinely biopsied. This may explain why the portion of the BE population associated with EAC was higher in our study population (3.4%).
Nonetheless, this study also has several important distinctions. First, the characteristics and risk of esophageal malignancy in the BE population became an important clinical issue. Unquestionably, endoscopic surveillance is important in detecting BE and associated malignancies for such BE populations with dysplasia [35] ; [36] . Identifying and focusing on high-risk patients in the general population would bring cost benefits for the current health care system. Second, current follow-up endoscopic BE surveillance for nondysplasia, low-grade dysplasia, and high-grade dysplasia were done every 3 years, 1 year, and 3 months, respectively [35] . In patients with BE and adenocarcinoma concurrently, most do not have typical reflux symptoms or erosive esophagitis, but present with almost alarm symptoms. Lump symptoms would be recognized by the patients themselves, and further endoscopic examination usually showed visible lumps or nodules that could be considered a more advanced lesion with invasion [33] ; [37] . Therefore, for these high-risk patients, it was important to detect EAC at an early stage.
In conclusion, our study found that BE-associated EAC mostly occurred in older men. In the group with BE-associated EAC, most of them were discovered due to alarm symptoms and meanwhile EAC had already developed. Further prospective study is needed to stratify the risk of disease progression in BE patients.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- [1] P. Sharma; Barretts esophagus; N Engl J Med, 361 (2009), pp. 548–556
- [2] P.J. de Jonge, M. van Blankenstein, C.W. Looman, M.K. Casparie, G.A. Meijer, E.J. Kuipers; Risk of malignant progression in patients with Barretts oesophagus: a Dutch nationwide cohort study; Gut, 59 (2010), pp. 1030–1036
- [3] F. Yousef, C. Cardwell, M.M. Cantwell, K. Galway, B.T. Johnston, L. Murray; The incidence of esophageal cancer and high-grade dysplasia in Barretts esophagus: a systematic review and meta-analysis; Am J Epidemiol, 168 (2008), pp. 237–249
- [4] F. Hvid-Jensen, L. Pedersen, A.M. Drewes, H.T. Sorensen, P. Funch-Jensen; Incidence of adenocarcinoma among patients with Barretts esophagus; N Engl J Med, 365 (2011), pp. 1375–1383
- [5] P. Sharma, K. McQuaid, J. Dent, R. Sampliner, S. Spechler, A. Cameron, et al.; A critical review of the diagnosis and management of Barretts esophagus: the AGA Chicago Workshop; Gastroenterology, 127 (2004), pp. 10–30
- [6] P.C. Enzinger, R.J. Mayer; Esophageal cancer; N Engl J Med, 349 (2003), pp. 2241–2252
- [7] A. Pennathur, A. Farkas, A.M. Krasinskas, P.F. Ferson, W.E. Gooding, M.K. Gibson, et al.; Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy; Ann Thorac Surg, 87 (2009), pp. 1048–1054 discussion 54–5
- [8] H.S. Lee, S.W. Jeon; Barrett esophagus in Asia: same disease with different pattern; Clin Endosc, 47 (2014), pp. 15–22
- [9] K.Y. Ho, T.K. Cheung, B.C. Wong; Gastroesophageal reflux disease in Asian countries: disorder of nature or nurture?; J Gastroenterol Hepatol, 21 (2006), pp. 1362–1365
- [10] M. Hongo, Y. Nagasaki, T. Shoji; Epidemiology of esophageal cancer: orient to occident. Effects of chronology, geography and ethnicity; J Gastroenterol Hepatol, 24 (2009), pp. 729–735
- [11] L.R. Lundell, J. Dent, J.R. Bennett, J.R. Bennett, A.L. Blum, D. Armstrong, J.P. Galmiche, et al.; Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification; Gut, 45 (1999), pp. 172–180
- [12] I. Hirano, N. Moy, M.G. Heckman, C.S. Thomas, N. Gonsalves, S.R. Achem; Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system; Gut, 62 (2013), pp. 489–495
- [13] P. Sharma, J. Dent, D. Armstrong, J.J. Bergman, L. Gossner, Y. Hoshihara, et al.; The development and validation of an endoscopic grading system for Barretts esophagus: the Prague C & M criteria; Gastroenterology, 131 (2006), pp. 1392–1399
- [14] Y.C. Lee, H.P. Wang, H.M. Chiu, S.C. Liao, S.P. Huang, Y.P. Lai, et al.; Comparative analysis between psychological and endoscopic profiles in patients with gastroesophageal reflux disease: a prospective study based on screening endoscopy; J Gastroenterol Hepatol, 21 (2006), pp. 798–804
- [15] P.H. Tseng, Y.C. Lee, H.M. Chiu, S.P. Huang, W.C. Liao, C.C. Chen, et al.; Prevalence and clinical characteristics of Barretts esophagus in a Chinese general population; J Clin Gastroenterol, 42 (2008), pp. 1074–1079
- [16] C.J. Kuo, C.H. Lin, N.J. Liu, R.C. Wu, J.H. Tang, C.L. Cheng; Frequency and risk factors for Barretts esophagus in Taiwanese patients: a prospective study in a tertiary referral center; Dig Dis Sci, 55 (2010), pp. 1337–1343
- [17] C. Yeh, C.T. Hsu, A.S. Ho, R.E. Sampliner, R. Fass; Erosive esophagitis and Barretts esophagus in Taiwan—a higher frequency than expected; Dig Dis Sci, 42 (1997), pp. 702–706
- [18] C.Y. Chang, M.B. Cook, Y.C. Lee, J.T. Lin, T. Ando, S. Bhatia, et al.; Current status of Barretts esophagus research in Asia; J Gastroenterol Hepatol, 26 (2011), pp. 240–246
- [19] M. Nilsson, R. Johnsen, W. Ye, K. Hveem, J. Lagergren; Prevalence of gastro-oesophageal reflux symptoms and the influence of age and sex; Scand J Gastroenterol, 39 (2004), pp. 1040–1045
- [20] G. Edgren, H.O. Adami, E. Weiderpass, O. Nyren; A global assessment of the oesophageal adenocarcinoma epidemic; Gut, 62 (2013), pp. 1406–1414
- [21] J.H. Rubenstein, N. Mattek, G. Eisen; Age- and sex-specific yield of Barretts esophagus by endoscopy indication; Gastrointest Endosc, 71 (2010), pp. 21–27
- [22] J. Lagergren, R. Bergstrom, A. Lindgren, O. Nyren; Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma; N Engl J Med, 340 (1999), pp. 825–831
- [23] D.A. Corley, A. Kubo, T.R. Levin, G. Block, L. Habel, G. Rumore, et al.; Race, ethnicity, sex and temporal differences in Barretts oesophagus diagnosis: a large community-based study, 1994-2006; Gut, 58 (2009), pp. 182–188
- [24] E.V. Robertson, M.H. Derakhshan, A.A. Wirz, Y.Y. Lee, J.P. Seenan, S.A. Ballantyne, et al.; Central obesity in asymptomatic volunteers is associated with increased intrasphincteric acid reflux and lengthening of the cardiac mucosa; Gastroenterology, 145 (2013), pp. 730–739
- [25] H. Pohl, K. Wrobel, C. Bojarski, W. Voderholzer, A. Sonnenberg, T. Rosch, et al.; Risk factors in the development of esophageal adenocarcinoma; Am J Gastroenterol, 108 (2013), pp. 200–207
- [26] S.J. Murphy, L.A. Anderson, H.R. Ferguson, B.T. Johnston, P.R. Watson, J. McGuigan, et al.; Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barretts esophagus; J Nutr, 140 (2010), pp. 1757–1763
- [27] T.-Y. Lee, H.-C. Lien, C.-S. Chang, M.-C. Wen; Barretts esophagus and severe reflux esophagitis share common pathophysiological characteristics among Chinese in Taiwan; Intern Med, 47 (2008), pp. 1767–1773
- [28] B. Westhoff, S. Brotze, A. Weston, C. McElhinney, R. Cherian, M.S. Mayo, et al.; The frequency of Barretts esophagus in high-risk patients with chronic GERD; Gastrointest Endosc, 61 (2005), pp. 226–231
- [29] R. Erichsen, D. Robertson, D.K. Farkas, L. Pedersen, H. Pohl, J.A. Baron, et al.; Erosive reflux disease increases risk for esophageal adenocarcinoma, compared with nonerosive reflux; Clin Gastroenterol H, 10 (2012), pp. 475–480
- [30] P.A.C. Gatenby, J.R. Ramus, C.P.J. Caygill, A. Charlett, M.C. Winslet, A. Watson; Treatment modality and risk of development of dysplasia and adenocarcinoma in columnar-lined esophagus; Dis Esophagus, 22 (2009), pp. 133–142
- [31] C. Tharavej, J.A. Hagen, J.H. Peters, G. Portale, J. Lipham, S.R. DeMeester, et al.; Predictive factors of coexisting cancer in Barretts high-grade dysplasia; Surg Endosc, 20 (2006), pp. 439–443
- [32] F.P. Peters, K.P. Brakenhoff, W.L. Curvers, W.D. Rosmolen, P. Fockens, F.J. ten Kate, et al.; Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barretts esophagus; Gastrointest Endosc, 67 (2008), pp. 604–609
- [33] O. Pech, L. Gossner, H. Manner, A. May, T. Rabenstein, A. Behrens, et al.; Prospective evaluation of the macroscopic types and location of early Barretts neoplasia in 380 lesions; Endoscopy, 39 (2007), pp. 588–593
- [34] M.A. Eloubeidi, D. Provenzale; Clinical and demographic predictors of Barretts esophagus among patients with gastroesophageal reflux disease: a multivariable analysis in veterans; J Clin Gastroenterol, 33 (2001), pp. 306–309
- [35] K.K. Wang, R.E. Sampliner, Practice Parameters Committee of the American College of Gastroenterology; Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barretts esophagus; Am J Gastroenterol, 103 (2008), pp. 788–797
- [36] W.K. Hirota, M.J. Zuckerman, D.G. Adler, R.E. Davila, J. Egan, J.A. Leighton, et al.; ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract; Gastrointest Endosc, 63 (2006), pp. 570–580
- [37] C. Bennett, N. Vakil, J. Bergman, R. Harrison, R. Odze, M. Vieth, et al.; Consensus statements for management of Barretts dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process; Gastroenterology, 143 (2012), pp. 336–346
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?