(Created page with "==Highlights== * HIV patients aviremic under antiretroviral therapy present with five different profiles of persistent immune activation. * One of these profiles is strongly l...") |
m (Scipediacontent moved page Draft Content 832916973 to Psomas et al 2016a) |
(No difference)
| |
Revision as of 11:13, 6 April 2017
Highlights
- HIV patients aviremic under antiretroviral therapy present with five different profiles of persistent immune activation.
- One of these profiles is strongly linked to marks of metabolic syndrome.
Research in context HIV-infected individuals under treatment present with a global activation of their immune system. We show that these patients may be clustered into five groups of immune activation. One of these groups presented with a high frequency of metabolic disorders known to favour cardiovascular and liver diseases. Our data suggest that particular types of immune activation might pave the way for particular chronic diseases. This might be the case in other situations of chronic immune activation, including aging. Unveiling the molecular links between immune activation and chronic diseases might provide with markers predictive of these diseases and with specific therapeutic targets.
Abstract
Immune activation in HIV-1-infected individuals is reduced under antiretroviral therapies, but persists, resulting in various morbidities. To better characterize this phenomenon, using a panel of 68 soluble and cell surface markers, we measured the level of activation in circulating CD4+ and CD8+ T cells, B cells, monocytes, NK cells, polynuclear and endothelial cells as well as of inflammation and fibrinolysis in 120 virologic responders over 45 years of age. As compared with age- and sex-matched uninfected individuals, we observed a persistence of activation in all the cell subpopulations analyzed, together with marks of inflammation and fibrinolysis. Two independent hierarchical clustering analyses allowed us to identify five clusters of markers that varied concurrently, and five patient groups, each with the same activation profile. The five groups of patients could be characterized by a marker of CD4+ T cell, CD8+ T cell, NK cell, monocyte activation or of inflammation, respectively. One of these profiles was strongly associated with marks of metabolic syndrome, particularly with hyperinsulinemia (OR 12.17 [95% CI 1.79–82.86], p = 0.011). In conclusion, our study unveils biomarkers linked to metabolic syndrome that could be tested as predictive markers, and opens the way to new therapeutic approaches tailored to each patient group.
Keywords
Hyperinsulinemia ; Cell activation ; Inflammation ; Coagulation ; Endothelium
1. Introduction
Today, about 90% of treated persons living with HIV are aviremic (Delaugerre et al., 2015 ). Yet, 6 to 24% of these virologic responders do not restore their CD4 count as they should, exposing them to the risk of immune deficiency-linked morbidities (Corbeau and Reynes, 2011 ). The main reason for that is the persistence of a state of hyperactivity of the immune system in these patients (Corbeau and Reynes, 2011 ). This residual immune activation also fuels the so-called non-AIDS-linked morbidities such as atherothrombosis, osteoporosis, metabolic syndrome, neurocognitive disorders, liver steatosis, kidney failure, frailty, and some types of cancer (Hunt, 2012 ). These comorbidities are now the major cause of morbi-mortality in treated patients (Lau et al., 2007 ).
HIV infection is characterized by the high level of immune activation induced in the human organism. Various causes may combine to provoke this immune hyperactivity (Younas et al., 2015 ). A first cause is the presence of the virus itself, detected by the immune system as a xenoantigen and a danger. For example, gp120 has recently been shown to activate monocytes and macrophages via TLR4 (Del Corno et al., 2016 ). A second cause is the infection process that triggers a DNA detection system resulting in inflammatory cytokine production (Doitsh et al., 2014 ). A third cause is the virus-induced deterioration of the gut barrier that opens the way for intestinal microbes to enter the human organism (Zevin et al., 2016 ). A fourth cause is the microbes that co-infect the organism (Boulougoura and Sereti, 2016 ), as for instance hepatitis (Kovacs et al., 2008 ) or herpes viruses (Sheth et al., 2008 ). A fifth set of causes is linked to some effects of HIV on the immune system itself: CD4+ T lymphopenia (Zonios et al., 2008 and Sereti et al., 2009 ), immunosenescence (Effros et al., 2003 ) and an imbalance in the CD4+ T cell subpopulations (Kanwar et al., 2010 ). Finally, metabolic dysregulation may also be a source of immune activation (Aounallah et al., 2016 , Dagenais-Lussier et al., 2015 and Palmer et al., 2016 ). For instance, lipid peroxidation may fuel monocyte activation (Zidar et al., 2015 ). Moreover, the oxidative stress in the course of HIV infection might increase inflammatory cytokines production (Chaudhri and Clark, 1989 ). Combined antiretroviral therapies (cART) reduce all the sources of immune activation without abolishing them (Palmer et al., 2008 , Guadalupe et al., 2003 , Gonzalez et al., 2009 and Stone et al., 2005 ). Consequently, immune activation is only partly downregulated in aviremic patients under treatment.
Previous works have shed a partial light on this phenomenon. The adaptative and the innate immune systems, including inflammation, are both involved. T cell activation has been particularly studied. Many authors have shown the persistence of marks of CD4+ and CD8+ T cell activation (Hunt et al., 2003 ), exhaustion (Cockerham et al., 2014 ), and senescence (Stone et al., 2005 ) under cART. The same is true for NK cells (Lichtfuss et al., 2012 and Mavilio et al., 2005 ). Likewise, B cells remain unquiet in virologic responders, as testified by the presence of hyperglobulinemia (De Milito et al., 2004 ). In the same type of patients, high peripheral blood levels of soluble CD14 (sCD14) and soluble CD163 (sCD163) have also been reported (Lichtfuss et al., 2012 , Burdo et al., 2011 , Rajasuriar et al., 2010 , Wallet et al., 2010 and Kamat et al., 2012 ), considered as signs of monocyte/macrophage hyperactivity. Even neutrophils present with marks of activation in HIV-infected subjects, including high cell surface expression of the program death ligand 1 PD-L1 (Bowers et al., 2014 ) and of the adhesion molecules CD11b and CD18, as well as low cell surface expression of CD62L (Elbim et al., 1994 ), and PD-L1 expression has been shown to remain elevated on ART (Bowers et al., 2014 ). Inflammation is also evident in HIV patients, and for instance the routine marker CRP and the soluble form of TNFα receptor II has been reported to be incompletely reduced under treatment (Brazille et al., 2003 , Aukrust et al., 1999 , French et al., 2009 , Calmy et al., 2009 , Guimaraes et al., 2008 , Regidor et al., 2011 , Baker et al., 2011 , van Vonderen et al., 2009 , Funderburg et al., 2010 and Shikuma et al., 2011 ). Various soluble markers have been used to monitor the activation of endothelial cells in the course of HIV infection, and some of them have been reported to be still increased after 12 years of ART (Rhönsholt et al., 2013 ). Finally, high levels of the main marker of fibrinolysis D-dimer have been observed (Kuller et al., 2008 , Rodger et al., 2009 and Neuhaus et al., 2010 ) that are not normalized under cART (Neuhaus et al., 2010 ).
Many studies have explored different aspects of this chronic immune, endothelial and coagulation activation, but we still lack a global picture of this phenomenon. Also, whether all patients have the same profile of activation or if various profiles may be distinguished remains unknown. This question is of major importance if we want to elucidate the specific role played by the different causes of immune activation, and for instance if some causes will favour some types of immune activation and other causes other types. It is also of importance if we want to have a better insight into the links between immune activation and comorbidities. A global analysis of immune activation might help us to assess if some profiles of immune activation favour specifically some morbidities. It is with this aim in mind, that we carried out an extensive characterization of the immune, endothelial and coagulation activation in HIV patients aviremic under treatment. This study enabled us to define five different profiles of immune activation in virologic responders.
2. Materials and methods
2.1. Study design
We carried out a cross-sectional observational study at two University Hospitals in France (Montpellier and Nîmes). Eligible patients were HIV-1-infected adults (age > 45 years) with pretherapeutic CD4 cell counts of 350 cells per μL or less, CD4 cell counts of 200 cells per μL or more in the last 6 months, and plasma viral load below 50 copies per mL for four time points each separated by 6 months while on a potent, stable combination antiretroviral regimen. Exclusion criteria were: pregnancy, breastfeeding, immunomodulatory therapy, anticancer chemotherapy, active hepatitis B or C virus infection, decompensated cirrhosis, and any non-infectious disease susceptible to impact on the immune system. The Ethics Committee of the University Hospital of Montpellier approved this study. All patients provided written informed consent.
2.2. Flow cytometry
Monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), energy-coupled dye (ECD), PE-Cyanine5.5 (PC5.5), PE-Cyanine7 (PC7), allophycocyanine (APC), APC/Alexa700, or APC/Alexa750 were purchased from Beckman Coulter. The antibodies were used in the following combinations; CD57-FITC/CD279-PE/CD45RA-ECD/C2-PC5.5/CD27-PC7/CD8-APC/CD4-APC700/CD3-APC750, CD8-APC/CD4-APC700/CD3-APC750/CD38-PE/HLADR-PC7/CD20-FITC, CD3-APC750/CD16 APC/HLADR-PC7/CD56-PC5.5/CD69-PE/CD57-FITC, CD4-FITC/CD45RA-ECD/CD25 PC7/FOXP3-APC/CD127-APC750. Whole blood collected into EDTA tubes was stained within 1 hour for 10 min at room temperature in the dark with cocktail of antibodies and fixed using IntraPrep Permeabilization Reagent Kit (Beckman Coulter) according to manufacturers protocol. Cells were run on a Navios flow cytometer and results were analyzed by using Kaluza software (Beckman Coulter). A minimum 20,000 lymphocytes were gated to analyze the subpopulations. Representative gating strategies and images of flow plots are shown in Supplementary Fig. 1 . We controlled the inter-run variability with the same batch of FlowSet Pro Beads (Beckman Coulter). The targets were defined on the samples settings (voltages). During the study, no voltage adjustment has been necessary to keep the beads into their respective defined targets.
2.3. Metabolic and soluble immunologic markers in peripheral blood
ELISA was used to quantify soluble TNF receptor I (sTNFRI), sCD14 and sCD163 (Quantikine, R&D systems), as well as tissue Plasminogen Activator, soluble Thrombomodulin, and soluble Endothelial Protein C Receptor (Asserachrom, Stago) in plasma collected into EDTA Vacutainer tubes (Becton Dickinson) and frozen. CRP and immunoglobulins were measured by turbidimetry in serum collected into SST II Vacutainer tubes, and D-dimers in blood collected into CTAD Vacutainer tubes (Becton Dickinson). Blood triglyceride, high-density lipoprotein (HDL) cholesterol, and insulin levels were quantified in serum collected into SST II Vacutainer tubes (Becton Dickinson) after 8 hours of fasting.
2.4. Statistical analysis
We used Wilcoxon tests to compare patients and donors, and Spearman rank correlations to evaluate the link between biomarkers with p -values multiplicity-adjusted by the False Discovery Rate method. We sorted the dataset with two independent hierarchical clustering analyses using respectively correlation and Euclidean distance to measure proximities/distances between markers and patients. The heatmap was generated using the heatmap function of the software R. The classifications used Ward (on the squared distance) as a metric. The distance was the euclidean for the patients and 1-abs (correlation) for markers. We used ANOVA results corrected by False Discovery Rate for multiple testing, to determine the frequency of activation markers significantly different for at least one group of patients with regards to the other ones. Logistic regressions were carried out in order to study the relationship between profiles of immune activation and metabolic syndrome. Univariate analyses were first performed, and an adjustment by the age was thereafter executed. To study the relationship between activation markers of various components of the immune, endothelial and coagulation systems, we performed spearman correlations, and a Principal Component Analysis (PCA) after standardization of our variables. PCA allowed us to analyze multiple correlated values simultaneously, and to represent it as a set of new orthogonal variables called principal components, linear combinations of the variables. Then, the analysis of the components was carried out in order to highlight the degree of correlation between the variables. Statistical analyses were carried out using SAS Enterprise Guide V4.3 (SAS Institute Inc.), and R V3.1.1 (The R Foundation for Statistical Computing). A p -value lower than 0.05 was considered statistically significant.
3. Funding source
University Hospitals of Nîmes and Montpellier. The funding sources were involved neither in the study design, in the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the article for publication.
4. Results
4.1. Study subjects
Between April 29 and September 17, 2014, we recruited 22 female and 98 male HIV virologic responders (Table 1 ). Ninety-five percent of them were Caucasians. For all patients included, mean CD4 cell count was 688 (SD 326) cells per μL with a mean CD4:CD8 ratio of 0.99 (SD 0.52). Mean duration of HIV infection was 17.2 (SD 7.4) years with a mean pretherapeutic CD4 cell count of 192 (SD 108) cells per μL. 29% were current smokers, and 19% former smokers. The percentages of patients presenting with hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), or Epstein-Barr virus (EBV) infection were 72%, 42%, 5%, 91%, and 98% respectively. Sex- and age-matched HIV-uninfected and HIV-infected, viremic, untreated caucasians were enrolled as negative and positive controls, respectively.
| Characteristic | HIV − | HIV + treated | HIV + untreated | |
|---|---|---|---|---|
| Number of individuals | 20 | 120 | 10 | |
| Age | Mean (± SD) | 54.6 (± 6.4) | 56.5 (± 8.0) | 52.8 (± 10.2) |
| Median (min–max) | 53.0 (46.0–69.0) | 55.0 (45.0–83.0) | 50.5 (36.0–68.0) | |
| Male sex | N (%) | 16 (80) | 98 (82) | 8 (80) |
| Caucasians | N (%) | 20 (100) | 114 (95) | 10 (100) |
| % CD4+ T cell | Mean (± SD) | ND | 34.7 (± 9.8) | 29.6 (± 10.7) |
| Median (min–max) | ND | 34.0 (15.0–62.0) | 28.0 (16.0–47.0) | |
| CD4 count (/mm3 ) | Mean (± SD) | ND | 688 (± 326) | 574 (± 283) |
| Median (min–max) | ND | 593 (243–2044) | 509 (166–1153) | |
| CD4:CD8 ratio | Mean (± SD) | ND | 0.99 (± 0.52) | 0.65 (± 0.33) |
| Median (min–max) | ND | 0.90 (0.20–3.30) | 0.60 (0.20–1.20) | |
| Pretherapeutic nadir CD4 count (cells/μL) | Mean (± SD) | NA | 192 (± 108) | 515 (± 304) |
| Median (min–max) | NA | 187 (1–458) | 442 (130–1153) | |
| Pretherapeutic viremia (RNA copies/ml) | Mean (± SD) | NA | 393,985 (± 1,314,356) | 58,833 (± 55,700) |
| Median (min–max) | NA | 104,156 (60–12,000,000) | 35,234 (1220–170,318) | |
| Years of HIV infection | Mean (± SD) | NA | 17.2 (± 7.4) | 1.1 (± 1.6) |
| Median (min–max) | NA | 18.0 (3.6–30.4) | 0.3 (0.1–4.2) | |
| Duration of viral suppression (months) | Mean (± SD) | NA | 102 (± 47) | NA |
| Median (min–max) | NA | 95 (25–217) | NA | |
| NRTI | N (%) | NA | 108 (90) | NA |
| NNRTI | N (%) | NA | 46 (38) | NA |
| Protease inhibitor | N (%) | NA | 61 (51) | NA |
| Integrase inhibitor | N (%) | NA | 34 (28) | NA |
| HBs Ag + | N (%) | ND | 3 (2) | 0 (0) |
| Anti-HBs Ab + | N (%) | ND | 53 (44) | 6 (60) |
| Anti-HBc Ab + | N (%) | ND | 50 (42) | 2 (20) |
| HCV coinfection | N (%) | ND | 6 (5) | 0 (0) |
| CMV coinfection | N (%) | ND | 109 (91) | 9 (90) |
| EBV coinfection | N (%) | ND | 118 (98) | 10 (100) |
| HAV coinfection | N (%) | ND | 87 (72) | 7 (70) |
| Systolic blood pressure (mmHg) | Mean (± SD) | ND | 128 (± 16) | 124 (± 14) |
| Median (min–max) | ND | 127 (100–181) | 128 (100–136) | |
| Diastolic blood pressure (mmHg) | Mean (± SD) | ND | 80 (± 11) | 82 (± 10) |
| Median (min–max) | ND | 80 (60–120) | 82 (60–94) | |
| Waist circumference (cm) | Mean (± SD) | ND | 91 (± 11) | 89 (± 10) |
| Median (min–max) | ND | 91 (65–128) | 85 (82–108) | |
| Lipodystrophy | N (%) | ND | 40 (33) | 0 (0) |
| Insulinemia (μUl/ml) | Mean (± SD) | ND | 11 (± 7) | 7 (± 5) |
| Median (min–max) | ND | 9 (1–45) | 7 (2–17) | |
| Triglyceridemia (mM/L) | Mean (± SD) | ND | 1.86 (± 1.75) | 1.55 (± 1.59) |
| Median (min–max) | ND | 1.57 (0.58–17.60) | 1.10 (0.64–5.70) | |
| HDL serum level (mM/L) | Mean (± SD) | ND | 1.45 (± 0.61) | 1.25 (± 0.55) |
| Median (min–max) | ND | 1.33 (0.16–4.20) | 1.17 (0.50–2.42) | |
4.2. Activation markers
We selected markers known to be modified in untreated HIV patients, and potentially in treated HIV patients (Younas et al., 2015 ). At the T lymphocyte surface, we chose the activation markers HLA-DR and CD38, as well as the inhibitory receptor Programmed cell Death 1 (PD-1 or CD279). CD45RA and CD27 were used to distinguish naïve and central memory from effector memory T cells. CD57 upregulation along with CD27 and/or CD28 loss were used to characterize T lymphocyte senescence. In addition to these markers we also measured CD38 overexpression to evaluate CD8+ T cell activation (Tuaillon et al., 2009 ). NK cell activation was monitored analyzing HLA-DR and CD69 expression, NK cell dysfunction CD56 loss, and NK cell senescence CD57 gain. B cell hyperactivity was assessed by quantifying IgG, IgA, and IgM in the serum. The plasma level of sCD14 and sCD163 were used as indicators of monocyte/macrophage activation, and that of sTNFRI and C-reactive protein (CRP) as indicators of inflammation. Endothelium activation was explored by looking for an increase in soluble Endothelial Protein C Receptor (sEPCR) and in tissue Plasminogen Activator (tPA), and for a decrease in soluble Thrombomodulin (sThrombomodulin) plasma levels. In a subgroup of patients, D-dimer plasma concentration was measured as well as CD64, CD62L, and PD-L1 polynuclear surface density.
4.3. Immune activation in virologic responders is global
As compared with HIV-negative controls, we observed in patients aviremic under cART an increase in the percentages of activated, HLA-DR+, CD4+ T cells (16 ± 8 and 22 ± 13, p = 0.015), of activated, HLA-DR+ (45 ± 15, 54 ± 18, p = 0.042), and senescent, CD57+, CD8+ T cells (18 ± 18, 32 ± 14, p = 0.001), and of activated, HLA-DR+ (33 ± 22, 52 ± 22, p = 0.002), dysfunctional, CD56- (8 ± 4, 11 ± 7, p = 0.030), and senescent, CD57+ (33 ± 16, 44 ± 18, p = 0.012) NK cells (Fig. 1 ). Yet, these percentages were lower than those observed in non-treated HIV-infected individuals. We also observed an increase in the plasma level of sCD163 (279 ± 158 ng/mL, 467 ± 282 ng/mL, p = 0.033), and sTNFRI (0.9 ± 0.3 ng/mL, 1.2 ± 0.5 ng/mL, p = 0.012), even though these levels were below those measured in treatment-naïve patients. CD64 polynuclear surface density was higher in treated patients than in healthy controls (1.0 ± 0.3 versus 0.8 ± 0.1 arbitrary units, p = 0.030). As compared with virologic responders (95 ± 56 ng/mL) sThrombomodulin concentration was higher in control subjects (142 ± 29 ng/mL, p = 0.008), but lower in viremic patients. Finally, IgG, IgA, IgM, and D-dimer plasma concentrations in aviremic patients were lower than in viremic patients, and higher than in noninfected controls (data not shown). Thus, all the components of the innate and adaptative immune system we tested, as well as the endothelium and the coagulation, appeared globally activated in the HIV-1-infected individuals we analyzed, even though they had been aviremic for years.
|
|
|
Fig. 1. Immune activation in virologic responders. Percentages of various cell populations and plasma levels of soluble markers in healthy donors (HIV-), treated (HIV+ ART+), and untreated (HIV+ ART–) HIV patients. Data are presented as mean values and 95% confidence intervals; p -values are shown. |
4.4. Links between markers within each component of the immune and endothelial systems
We looked for correlations between the various markers within each cell subpopulation. In CD4+ T cells (Table 2 ), expression of the activation marker HLA-DR was linked to that of the inhibitory receptor PD-1, and of markers of senescence (CD57 expression with and without loss of CD28 and/or CD27). In CD8+ T cells (Table 3 ), we found no correlation between HLA-DR and CD38 expression. Moreover, in these lymphocytes, HLA-DR expression, but not that of CD38, was linked to markers of senescence. Of note, in CD8+ T cells, PD-1 expression was linked neither to these two markers of activation, nor to markers of senescence. In NK cells (Table 4 ), the two activation markers HLA-DR and CD69 were linked together but not to the marker of senescence CD57, but this latter was linked to the marker of dysfunction CD56-. Of note, the levels of the monocyte/macrophage activation markers sCD14 and sCD163 were not linked together (r = 0.087, p = 0.344). By contrast, the plasma concentrations of IgG and IgA (r = 0.424, p < 0.001), and IgG and IgM (r = 0.352, p < 0.001) were linked together.
| % CD4+ T cells HLA-DR+ | % CD4+ T cells PD-1+ | % CD4+ T cells CD57+ | % CD4+ T cells CD57+CD28– | % CD4+ T cells CD57+CD28–CD27– | |
|---|---|---|---|---|---|
| % CD4+ T cells HLA-DR+ | r = 0.393p < 10− 4 | r = 0.585p < 10− 4 | r = 0.584p < 10− 4 | r = 0.542p < 10− 4 | |
| % CD4+ T cells PD-1+ | r = 0.375p < 10− 4 | r = 0.374p < 10− 4 | r = 0.326p < 10− 4 | ||
| % CD4+ T cells CD57+ | r = 0.998p < 10− 4 | r = 0.976p < 10− 4 | |||
| % CD4+ T cells CD57+CD28– | r = 0.976p < 10− 4 | ||||
| % CD4+ T cells CD57+CD28–CD27– |
| % CD8+ T cells HLA-DR+ | % CD8+ T cells CD38+ | % CD8+ T cells CD38hi | % CD8+ T cells HLA-DR+CD38+ | % CD8+ T cells PD1+ | % CD8+ T cells CD57+ | % CD8+ T cells CD57+CD28– | % CD8+ T cells CD57+CD28–CD27– | |
|---|---|---|---|---|---|---|---|---|
| % CD8+ T cells HLA-DR+ | r = 0.615p < 10− 4 | r = 0.503p < 10− 4 | r = 0.494p < 10− 4 | r = 0.462p < 10− 4 | ||||
| % CD8+ Tcells CD38+ | r = 0.598p < 10− 4 | r = 0.443p < 10− 4 | ||||||
| % CD8+ T cells CD38hi | r = 0.354p < 10− 4 | |||||||
| % CD8+ T cells HLA-DR+CD38+ | r = 0.506p < 10− 4 | r = 0.493p < 10− 4 | r = 0.436p < 10− 4 | |||||
| % CD8+ T cells PD-1+ | ||||||||
| % CD8+ T cells CD57+ | r = 0.996p < 10− 4 | r = 0.905p < 10− 4 | ||||||
| % CD8+ T cells CD57+CD28– | r = 0.907p < 10− 4 | |||||||
| % CD8+ T cells CD57+CD28–CD27– |
| % NK cells HLA-DR + | % NK cells CD69 + | % NK cells HLA-DR+CD69+ | % NK cells CD56- | % NK CD57+ | |
|---|---|---|---|---|---|
| % NK cells HLA-DR+ | r = − 0.501p < 10− 4 | r = 0.395p < 10− 4 | |||
| % NK cells CD69+ | r = 0.270p = 0.0029 | ||||
| % NK cells HLA-DR+CD69+ | r = − 0.335p = 0.0479 | ||||
| % NK cells CD56- | r = 0.270p = 0.0002 | ||||
| % NK cells CD57+ |
4.5. Correlations between the various markers of the immune, endothelial, and coagulation activations
As we observed a hyperactivity in all the components we analyzed, we wondered whether the level of activity of some of these components were linked, either because their activation results from common causes and/or because they induce each other. To answer this question, we systematically looked for correlations between markers characterizing the activation of the various components of the immune, endothelial and coagulation systems (Table 5 ). As characteristic markers, we chose HLA-DR expression on CD4+ T cells, CD38 overexpression on CD8+ T cells, CD69 expression on NK cells, CD62L expression on polynuclear cells, sCD14, IgG, tPA, D-dimer and sTNFRI plasma levels for monocyte, B cell, endothelial, coagulation activation and inflammation, respectively. The only links we uncovered was between HLA-DR expression on CD4+ T cells and sCD14 level (r = 0.200, p = 0.028) on one hand, and sTNFRI and D-dimer levels (r = 0.215, p = 0.011) on the other hand.
| % CD4+ T cells HLA-DR+ | % CD8+ T cells CD38hi | % NK cells CD69+ | CD62L polynuclear cell surface density | sCD14 | IgG | sTNFR I | tPA | D-dimer | |
|---|---|---|---|---|---|---|---|---|---|
| % CD4+ T cells HLA-DR+ | – | r = 0.200p = 0.0284 | |||||||
| % CD8+ T cells CD38hi | |||||||||
| % NK cells CD69+ | |||||||||
| CD62L polynuclear cell surface density | |||||||||
| sCD14 | |||||||||
| IgG | |||||||||
| sTNFR I | – | r = 0.215p = 0.0107 | |||||||
| tPA | |||||||||
| D-dimer |
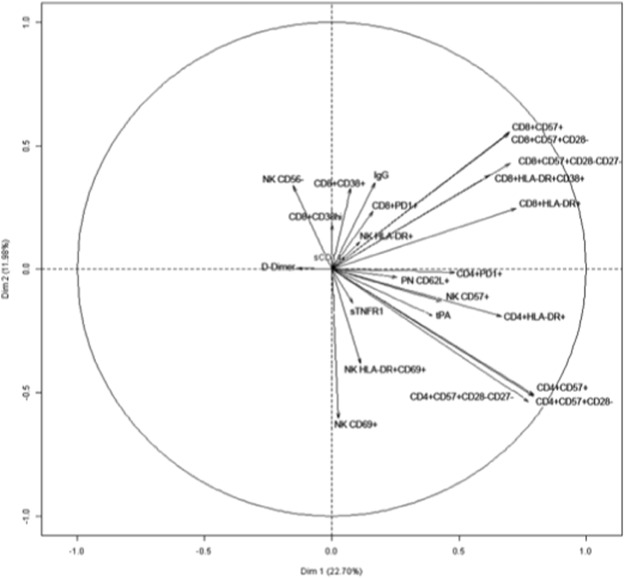
In order to comprehensively analyze more globally correlations between the various activation markers and to obtain a visual representation, a PCA was computed, after standardization of the variables (Fig. 2 ). We decided to keep two principal components after performing an elbow test. These two components represented about 35% of the models variance (22.70 + 11.96). The results corroborated the conclusions above. For instance, in CD4+ T cells, we found a strong correlation between markers of senescence (CD57 expression with and without loss of CD28 and/or CD27), and of activation (HLA-DR) and PD-1. By contrast, D-dimer levels appeared not to be linked to CD4+ T cell and CD8+ T cell markers.
|
|
|
Fig. 2. Variables factor map resulting from Principal Component Analysis. The variables are represented by arrows. The elbow test was carried out in order to select the number of components to consider. This was done by plotting the components' eigenvalues according to their size and analyzing the point in the graph where the slope goes from “steep” to “flat” in order to keep only the components that are placed before the elbow, which were 2 in our case. The length of each of these arrows depends on the correlation of the variable with the component. Highly positively correlated variables are represented by arrows close to each other. Strongly negatively correlated variables are represented by arrows diametrically opposed. |
4.6. Identification of different profiles of immune activation in patients aviremic under cART
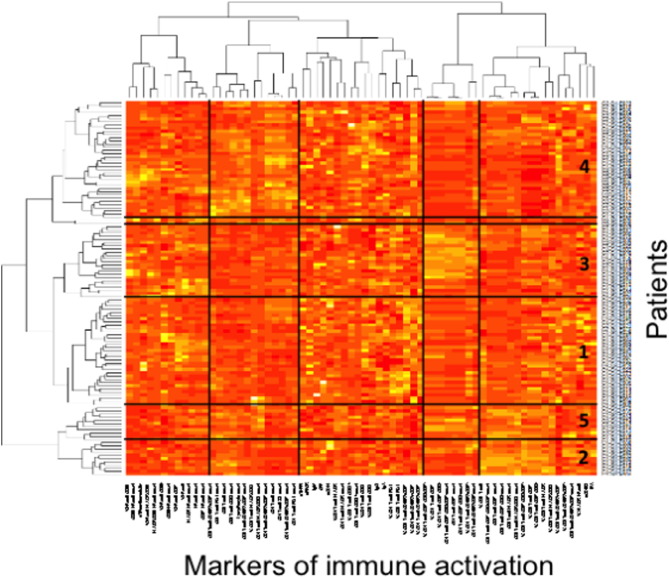
To test the hypothesis of the existence of different profiles of immune activation in patients aviremic under treatment, we sorted the patients and the 68 markers of lymphocyte, NK cell, monocyte, endothelium activation and inflammation by two independent hierarchical clustering analyses (Fig. 3 ). This clustering outlined the correlations, or the absence thereof, existing between some markers. Some of these correlations were expected. For instance the correlation between the percentages of CD8+ T cells positive for HLA-DR and for CD57 shown in Table 3 is illustrated by their proximity in the heatmap (8th and 10th markers from the right end). This clustering also unveiled the unexpected absence of links between other markers, such as between sCD14 and sCD163, as previously mentioned, situated far apart from each other in the heatmap. It also showed unexpected links between other markers. For instance, the marker of NK activation HLA-DR was linked to the marker of endothelium activation tPA (r = 0.261, p = 0.004). On the other hand, tPA appeared also linked to monocyte/macrophage activation as estimated by the level of sCD163 (r = 0.244, p = 0.007).
|
|
|
Fig. 3. Virologic responders present with different immune activation profiles. Heatmap showing the hierarchical clustering of the activation markers (vertical) as well as of the virologic responders according to their profile of activation (horizontal). Each group number is indicated. |
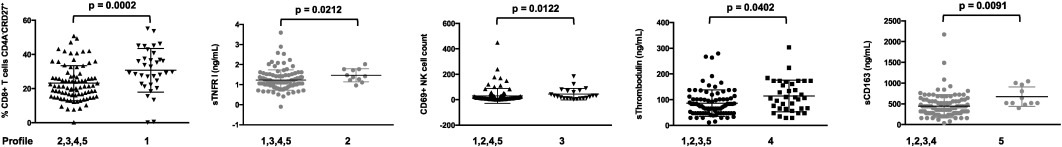
The hierarchical clustering analysis also identified five groups of patients, presenting with very different profiles of immune activation, and 2 outliers (Fig. 3 ). The levels of more than 80% of the markers were on average significantly different for at least one group of patients with regards to the other ones. The diversity of the activation profiles was evident when selected activation markers were analyzed (Fig. 4 ). As compared with the other groups, group 1 (T8 profile) was characterized by a high percentage of central memory (CD45RA-CD27+) CD8+ T cells (31 ± 13 versus 23 ± 10%, p = 0.0007), group 2 (inflammation profile) by a high level of the inflammation marker sTNFRI (1.5 ± 0.3 versus 1.2 ± 0.5 mg/L, p = 0.040), and group 3 (NK profile) by a high number of activated, CD69+, NK cells (41 ± 43 versus 31 ± 60 cells/μL, p = 0.033). Group 4 (T4 profile) had the highest proportion of CD4+ T cells expressing CD38 (65 ± 9% versus 55 ± 12%, p < 0.001), and group 5 (monocyte profile) the highest level of the monocyte activation marker sCD163 (671 ± 520 ng/mL versus 446 ± 279 ng/mL, p = 0.009). To simplify the identification of the five profiles of immune activation, we looked for a small number of markers able to characterize each patient group. Using only 8 biomarkers, we were able to classify the patients in the five groups with an error rate of 8.5%.
|
|
|
Fig. 4. Characterization of the five different immune activation profiles. Differences in the levels of key activation markers between each group of patients and the other groups are represented. Data are presented as mean values and 95% confidence intervals; p -values are shown. |
4.7. Relationship between a profile of immune activation and metabolic syndrome
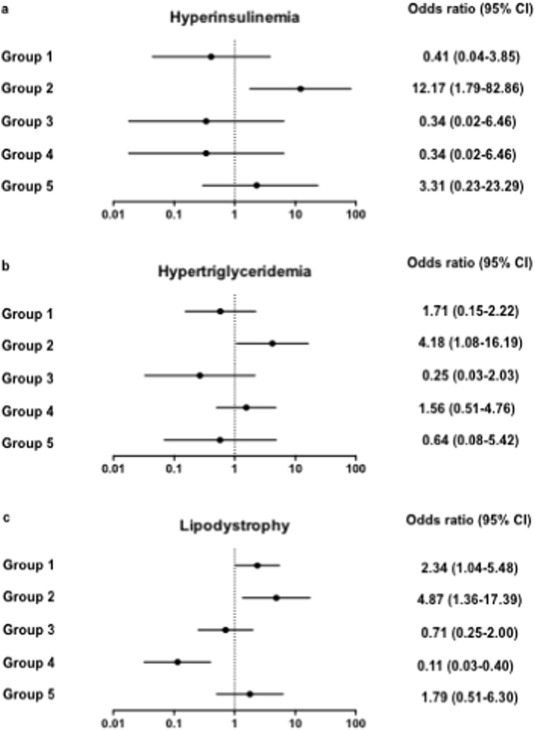
According to various studies, the frequency of metabolic syndrome has been reported as 14%–26% (Tebas, 2008 ) in HIV patients. This syndrome is characterized by the presence of at least 3 of the following components: hypertension, elevated waist circumference or waist-hip ratio, impaired glucose tolerance, and high triglyceride and/or low HDL cholesterol (Alberti et al., 2009 ). As the metabolic syndrome has been linked to immune activation (Esser et al., 2014 ), we looked for a relationship between this non-AIDS-linked morbidity and the immunological profiles we defined, by carrying out logistic regressions. Univariate analyses showed that, as compared with the other groups, patient group 2 presented with higher mean systolic and diastolic blood pressures, higher waist circumference, and lower HDL cholesterol levels, but these differences did not reach significance. The frequency of hyperinsulinemia (insulinemia > 24.9 mIU/L; OR 12.17 [95% CI 1.79–82.86], p = 0.011, Fig. 5 A), and of high (> 2.85 mM) triglyceridemia (OR 4.18 [95% CI 1.08–16.19], p = 0.038, Fig. 5 B) was significantly elevated in group 2 as compared with the other groups. Moreover, the proportion of patients presenting with lipodystrophia was also higher in group 2 than in the other groups (OR 4.87 [95% CI 1.36–17.39], p = 0.015, Fig. 5 C). Thus, the immune activation profile 2 was linked to major marks of one of non-AIDS comorbidities, the metabolic syndrome. Univariate analysis showed that, as compared with the other groups, patients in group 2 were slightly older (OR 1.08 [95% CI 1.01–1.16], p = 0.023). A multivariate analysis, adjusted with age as a covariate, still highlighted the risk of lipodystrophia (OR 4.37 [95% CI 1.17–16.33], p = 0.028) and a risk of hyperinsulinemia that was even higher (OR 17.06 [95% CI 2.14–135.60], p = 0.007) in the group 2.
|
|
|
Fig. 5. Link between immune activation profile 2 and marks of metabolic syndrome. Odd ratios relating each profile of immune activation to risk of hyperinsulinemia (a), hypertriglyceridemia (b), and lipodystrophy (c). Data are presented as OR and 95% confidence intervals. |
5. Discussion
In this study, we report a global evaluation of the state of activation of the immune, endothelial and coagulation systems in 120 treated patients aviremic for at least 2 years. A first conclusion is that these patients overall present with a generalized hyperactivity of these systems.
A second conclusion is that many markers of the activation of a cell subpopulation are not equivalent. Analyzing intra-subpopulation correlations, we noticed that HLA-DR expression and CD38 overexpression on CD8+ T cells were not correlated (Table 3 ). This is in line with the fact that these markers may not be coexpressed in vitro (Chadburn et al., 1992 ) and in vivo (Espinosa et al., 2013 ). It is also in agreement with the initial observation that CD38, but not HLA-DR, is a predictive marker of the disease progression (Giorgi et al., 1993 ). Clearly, the expression of these two markers is largely independent, probably driven by different mechanisms of activation, and leading to different consequences. Accordingly, CD8+ T cell senescence, as indicated by CD57 appearance and CD27 and/or CD28 loss, correlated with HLA-DR, but not with CD38 expression (Table 3 ). Strikingly, PD-1 expression was linked to none of these two activation markers (Table 3 ). This emphasizes the notion that the percentage of CD8+ T cells positive for PD-1 is dependent on the stage of differentiation, PD-1 being mostly expressed at an early and intermediate stage of differentiation, rather than on the level of activation (Sauce et al., 2007 ). Indeed, in our study, the percentage of CD8+ T cells expressing PD-1 was very strongly (data not shown) correlated with the percentage of CD8+ T cells CD45RA-CD27+. Consequently, alternative markers need to be used to measure exhaustion, as for instance CD160 (Vigano et al., 2014 ). A striking observation is also that sCD14 and sCD163 are situated far apart in the biomarker clustering. The absence of correlation between these two monocyte/macrophage activation markers has been reported elsewhere (Patro et al., 2016 ). This is in line with the fact that sCD163, but not sCD14, has been correlated with HIV load, and may normalize under antiretroviral therapy (Castley et al., 2014 ). Likewise, sCD163, but not sCD14, has been linked to the level of anti-CMV IgG (Hodowanec et al., 2015 ) and to liver fibrosis (Mueller et al., 2015 ). Altogether these data emphasize the idea that these two markers likely do not reflect the same activation pathway.
Another important finding of our study is that the various pathways we analyzed are not often activated concurrently. In the individuals we examined, most of the components of the immune, endothelial and coagulation systems, as evaluated by selected markers, appear to be independently activated. This raised the interesting hypothesis that virologic responders might present with different profiles of activation.
The ascendant hierarchical classification enabled us to cluster activation markers that fluctuate in the same way. Apart from some expected correlations, this classification uncovered some unexpected ones. Indeed, to our knowledge, correlations between NK and endothelium activation had never been previously reported in HIV infection. The fact that activated endothelial cells may release fractalkine, a chemokine known to activate NK cells (Robinson et al., 2003 ) might be a mechanism warranting further study. Likewise, no links between monocyte/macrophage and endothelium activation had been described so far in HIV patients. The fact that tPA has been reported to induce microglial activation via surface annexin II (Siao and Tsirka, 2002 ) might account for the correlation between tPA and sCD163 we uncovered. These examples highlight the interest of our global approach that may unveil interactions between the components of the immune, endothelial and coagulation systems.
Via this global approach we were able to cluster the patients into five different immune activation profiles. The diversity of these profiles is illustrated by the fact that over 80% of the markers are variable among the patient groups. Moreover, each of these groups can be distinguished by a different immune activation marker. As various factors, including residual HIV production, microbial translocation, coinfections, metabolic disorders, CD4+ T cell lymphopenia, immunosenescence, and Treg deregulation, may cause immune and endothelial activation, it is tempting to speculate that these five profiles are the result of different combinations of these etiologic factors. To test this possibility, we are currently measuring markers specific for these causes of activation in the same cohort of patients in order to look for correlations between these different causes and the five profiles of activation. To evaluate whether some coinfections might be coresponsible for the immune activation profile 2, we looked for a link between markers of these co-infections and this biomarker profile. As compared with the other patient groups, we did not find any increase in the frequency of either HAV, HBV, HCV or CMV seropositivity, or EBV load in patient group 2.
It is also possible that each activation profile fuels preferentially some comorbidities as compared with the other profiles. In line with this hypothesis, we observed a significant correlation between one of the patient group (group 2) and three hallmarks of the metabolic syndrome, hypertriglyceridemia, lipodystrophia and hyperinsulinemia. Of note, a characteristic of patients in group 2 is the high level of sTNFRI, a marker of TNFα production. Interestingly, TNFα has been reported to inhibit insulin signalling via the phosphorylation of the Insulin Receptor Substrate 1 (Hotamisligil et al., 1996 , Yin et al., 1998 and Aguirre et al., 2000 ), an effect that favours the development of metabolic syndrome (Alberti et al., 2009 ). Moreover, the plasma level of TNFα has been reported to be correlated with the metabolic syndrome (Shoelson et al., 2006 ).
Our study has some limitations. First, the prevalence of sexually transmitted diseases in patients and the CMV status as well as the proportion of substance abusers in the negative controls were unknown. These factors might confound the comparisons between healthy donors and HIV adults. It must also be noted that it is a cross-sectional study. This type of study design enables to show differences in immune activation profiles and correlations with a comorbidity. Yet, a longitudinal follow-up will be needed to test the predicting value of the immune activation profile of group 2.
From a more general point of view, it will be of interest to look for correlations between other comorbidities and the immune activation profiles uncovered by the present study. If we establish correlations between other comorbidities and some immune activation profiles, the simple signature of eight markers we have shown to be able to characterize each profile could be tested for its ability to predict some morbidities driven by immune/endothelial activation. Of practical interest, the identification of this signature requires a single flow cytometry tube. In the future, such a signature might enable to tailor prevention and early diagnosis of non-AIDS-linked morbidities to each patient. Unveiling links between some causes of immune activation and some immune activation profiles, and between some immune activation profiles and some comorbidities could also have therapeutic implications. Uncovering molecular mechanisms responsible for a causative link between a profile of immune activation and atherothrombosis, liver steatosis, osteoporosis or neurocognitive impairment could provide us with new therapeutic targets to prevent and attenuate these chronic diseases. Moreover, uncovering the causes (HIV and non-HIV infection, microbial translocation, metabolic dysregulation, CD4+ T cell loss, immunosenescence, and Treg dysfunction) fueling the immune activation profile of each patient could open the way to an etiologic treatment.
As these pathogenic pathways are probably not specific for HIV infection but relevant to any situation of chronic immune activation, including aging, and given the frequency of these comorbidities, these observations are of general interest. It is for instance striking that HIV infection and the autoimmune disease rheumatoid arthritis share many common comorbidities, including bone loss, atherosclerosis, metabolic syndrome, and frailty (Weyand et al., 2014 ). Thus, our approach could improve our knowledge on the pathogenic consequences of chronic immune activation, on the pathophysiology of the major chronic diseases it fuels, as well as on the prevention of these diseases, their early diagnosis and treatment.
The following is the supplementary data related to this article.
Fig. 1.
Flow cytometry analysis of lymphocyte populations from whole blood. First, doublets were excluded, and alive lymphocytes were gated based on FSC and SSC. (a) T lymphocytes were identified using CD3. CD4+ and CD8+ subsets were then distinguished within the T population. Naïve, central memory, effector and terminally differentiated CD4+ T cells were characterized based on CD45RA and CD27 expression. Within the CD4+ T lymphocytes, activated cells were identified using HLA-DR and CD38, exhausted cells using PD-1, and senescent cells using CD57 and CD28 (and CD27, not shown). (b) NK cells were identified using CD3 and CD16. Within the NK population, activated cells were identified using HLA-DR and CD69, dysfunctional cells using CD56, and senescent cells using CD57.
Conflicts of Interest
None.
Authors Contributions
Christina PSOMAS contributed to the conception and design of the study, the patients' enrollment, acquired, analyzed and interpreted data. Mehwish YOUNAS contributed to the conception and design of the flow cytometry study, acquired, analyzed and interpreted cell surface and soluble markers data. Renaud CEZAR, Pierre PORTALES, Edouard TUAILLON, Jean-François ELIAOU contributed to the conception and design of the flow cytometry study, acquired, analyzed and interpreted cell surface markers. Christelle REYNES, Robert SABATIER, Grégory MARIN, Nicolas NAGOT contributed to the conception and design of the statistical study, acquired, analyzed and interpreted statistical data. Adeline GUIGUES acquired and analyzed soluble markers data. Corinne MERLE, Nadine ATOUI, Céline FERNANDEZ, Vincent LE MOING, Claudine BARBUAT, Albert SOTTO acquired, analyzed and interpreted clinical data. Jacques REYNES contributed to the conception and design of the study, analyzed and interpreted data. Pierre CORBEAU contributed to the conception and design of the study, analyzed and interpreted data, and wrote the first draft of the manuscript. All authors revised and approved the final version.
Acknowledgments
We are grateful to the persons who volunteered for this study, and to Mariella Lomma for the critical reading of the manuscript. The study was funded by the university hospitals of Montpellier and Nîmes, and by the ANRS.
References
- Aguirre et al., 2000 V. Aguirre, T. Uchida, L. Yenush, R. Davis, M.F. White; The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307); J. Biol. Chem., 275 (12) (2000), pp. 9047–9054
- Alberti et al., 2009 K.G. Alberti, et al.; Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity; Circulation, 120 (16) (2009), pp. 1640–1645
- Aounallah et al., 2016 M. Aounallah, et al.; Current topics in HIV pathogenesis, part 2: inflammation drives a Warburg-like effect on the metabolism of HIV-infected subjects; Cytokine Growth Factor Rev., 28 (2016), pp. 1–10
- Aukrust et al., 1999 P. Aukrust, et al.; Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure; J. Infect. Dis., 179 (1) (1999), pp. 74–82
- Baker et al., 2011 J.V. Baker, et al.; Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection; J. Acquir. Immune Defic. Syndr., 56 (1) (2011), pp. 36–43
- Boulougoura and Sereti, 2016 A. Boulougoura, I. Sereti; HIV infection and immune activation: the role of coinfections; Curr. Opin. HIV AIDS, 11 (2) (2016), pp. 191–200
- Bowers et al., 2014 N.L. Bowers, et al.; Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway; PLoS Pathog., 10 (3) (2014), p. e1003993
- Brazille et al., 2003 P. Brazille, et al.; Decreases in plasma TNF-alpha level and IFN-gamma mRNA level in peripheral blood mononuclear cells (PBMC) and an increase in IL-2 mRNA level in PBMC are associated with effective highly active antiretroviral therapy in HIV-infected patients; Clin. Exp. Immunol., 131 (2) (2003), pp. 304–311
- Burdo et al., 2011 T.H. Burdo, et al.; Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy; J. Infect. Dis., 204 (1) (2011), pp. 154–163
- Calmy et al., 2009 A. Calmy, et al.; HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial; AIDS, 23 (8) (2009), pp. 929–939
- Castley et al., 2014 A. Castley, et al.; Elevated plasma soluble CD14 and skewed CD16 + monocyte distribution persist despite normalisation of soluble CD163 and CXCL10 by effective HIV therapy: a changing paradigm for routine HIV laboratory monitoring?; PLoS One, 9 (12) (2014), p. e115226
- Chadburn et al., 1992 A. Chadburn, G. Inghirami, D.M. Knowles; The kinetics and temporal expression of T-cell activation-associated antigens CD15 (LeuM1), CD30 (Ki-1), EMA, and CD11c (LeuM5) by benign activated T cells; Hematol. Pathol., 6 (4) (1992), pp. 193–202
- Chaudhri and Clark, 1989 G. Chaudhri, I.A. Clark; Reactive oxygen species facilitate the in vitro and in vivo lipopolysaccharide-induced release of tumor necrosis factor; J. Immunol., 143 (4) (1989), pp. 1290–1294
- Cockerham et al., 2014 L.R. Cockerham, et al.; Programmed death-1 expression on CD4 + and CD8 + T cells in treated and untreated HIV disease; AIDS, 28 (12) (2014), pp. 1749–1758
- Corbeau and Reynes, 2011 P. Corbeau, J. Reynes; Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection; Blood, 117 (21) (2011), pp. 5582–5590
- Dagenais-Lussier et al., 2015 X. Dagenais-Lussier, et al.; Current topics in HIV-1 pathogenesis: the emergence of deregulated immuno-metabolism in HIV-infected subjects; Cytokine Growth Factor Rev., 26 (6) (2015), pp. 603–613
- De Milito et al., 2004 A. De Milito, et al.; Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection; Blood, 103 (6) (2004), pp. 2180–2186
- Del Corno et al., 2016 M. Del Corno, et al.; HIV-1 gp120 signaling through TLR4 modulates innate immune activation in human macrophages and the biology of hepatic stellate cells; J. Leukoc. Biol. (2016) http://dx.doi.org/10.1189/jlb.4A1215-534R
- Delaugerre et al., 2015 C. Delaugerre, et al.; Significant reduction in HIV virologic failure during a 15-year period in a setting with free healthcare access; Clin. Infect. Dis., 60 (3) (2015), pp. 463–472
- Doitsh et al., 2014 G. Doitsh, et al.; Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection; Nature, 505 (7484) (2014), pp. 509–514
- Effros et al., 2003 R.B. Effros, Z. Cai, P.J. Linton; CD8 T cells and aging; Crit. Rev. Immunol., 23 (1–2) (2003), pp. 45–64
- Elbim et al., 1994 C. Elbim, et al.; Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming; Blood, 84 (8) (1994), pp. 2759–2766
- Espinosa et al., 2013 E. Espinosa, D.P. Romero-Rodriguez, M.T. Cantoral-Diaz, G. Reyes-Teran; Transient expansion of activated CD8(+) T cells characterizes tuberculosis-associated immune reconstitution inflammatory syndrome in patients with HIV: a case control study; J. Inflamm., 10 (2013), p. 21
- Esser et al., 2014 N. Esser, S. Legrand-Poels, J. Piette, A.J. Scheen, N. Paquot; Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes; Diabetes Res. Clin. Pract., 105 (2) (2014), pp. 141–150
- French et al., 2009 M.A. French, M.S. King, J.M. Tschampa, B.A. da Silva, A.L. Landay; Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4 + T cells; J. Infect. Dis., 200 (8) (2009), pp. 1212–1215
- Funderburg et al., 2010 N. Funderburg, et al.; Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4 + cell rises in HIV-infected patients; PLoS One, 5 (10) (2010), p. e13188
- Giorgi et al., 1993 J.V. Giorgi, et al.; Elevated levels of CD38 + CD8 + T cells in HIV infection add to the prognostic value of low CD4 + T cell levels: results of 6 years of follow-up. The Los Angeles center, multicenter AIDS cohort study; J. Acquir. Immune Defic. Syndr., 6 (8) (1993), pp. 904–912
- Gonzalez et al., 2009 V.D. Gonzalez, et al.; High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment; J. Virol., 83 (21) (2009), pp. 11407–11411
- Guadalupe et al., 2003 M. Guadalupe, et al.; Severe CD4 + T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy; J. Virol., 77 (21) (2003), pp. 11708–11717
- Guimaraes et al., 2008 M.M. Guimaraes, et al.; High-sensitivity C-reactive protein levels in HIV-infected patients treated or not with antiretroviral drugs and their correlation with factors related to cardiovascular risk and HIV infection; Atherosclerosis, 201 (2) (2008), pp. 434–439
- Hodowanec et al., 2015 A. Hodowanec, et al.; Soluble CD163 but not soluble CD14 is associated with cytomegalovirus immunoglobulin G antibody levels in virologically suppressed HIV + individuals; J. Acquir. Immune Defic. Syndr., 70 (5) (2015), pp. e171–e174
- Hotamisligil et al., 1996 G.S. Hotamisligil, et al.; IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance; Science, 271 (5249) (1996), pp. 665–668
- Hunt, 2012 P.W. Hunt; HIV and inflammation: mechanisms and consequences; Curr. HIV/AIDS Rep., 9 (2) (2012), pp. 139–147
- Hunt et al., 2003 P.W. Hunt, et al.; Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy; AIDS, 17 (13) (2003), pp. 1907–1915
- Kamat et al., 2012 A. Kamat, et al.; Monocyte Activation Markers in Cerebrospinal Fluid Associated With Impaired Neurocognitive Testing in Advanced HIV Infection; J. Acquir. Immune Defic. Syndr., 60 (3) (2012), pp. 234–243
- Kanwar et al., 2010 B. Kanwar, D. Favre, J.M. McCune; Th17 and regulatory T cells: implications for AIDS pathogenesis; Curr. Opin. HIV AIDS, 5 (2) (2010), pp. 151–157
- Kovacs et al., 2008 A. Kovacs, et al.; CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus; J. Infect. Dis., 197 (10) (2008), pp. 1402–1407
- Kuller et al., 2008 L.H. Kuller, et al.; Inflammatory and coagulation biomarkers and mortality in patients with HIV infection; PLoS Med., 5 (10) (2008), p. e203
- Lau et al., 2007 B. Lau, S.J. Gange, R.D. Moore; Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4 + counts greater than 200 cells/mm3; J. Acquir. Immune Defic. Syndr., 44 (2) (2007), pp. 179–187
- Lichtfuss et al., 2012 G.F. Lichtfuss, et al.; Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation; J. Immunol., 189 (3) (2012), pp. 1491–1499
- Mavilio et al., 2005 D. Mavilio, et al.; Characterization of CD56 −/CD16 + natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals; Proc. Natl. Acad. Sci. U. S. A., 102 (8) (2005), pp. 2886–2891
- Mueller et al., 2015 J.L. Mueller, et al.; Circulating soluble CD163 is associated with steatohepatitis and advanced fibrosis in nonalcoholic fatty liver disease; Clin. Transl. Gastroenterol., 6 (2015), p. e114
- Neuhaus et al., 2010 J. Neuhaus, et al.; Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection; J. Infect. Dis., 201 (12) (2010), pp. 1788–1795
- Palmer et al., 2008 S. Palmer, et al.; Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy; Proc. Natl. Acad. Sci. U. S. A., 105 (10) (2008), pp. 3879–3884
- Palmer et al., 2016 C.S. Palmer, C.L. Cherry, I. Sada-Ovalle, A. Singh, S.M. Crowe; Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis; EBioMedicine, 6 (2016), pp. 31–41
- Patro et al., 2016 S.C. Patro, et al.; Antiretroviral therapy in HIV-1-infected individuals with CD4 count below 100 cells/mm3 results in differential recovery of monocyte activation ; J. Leukoc. Biol. (2016) http://dx.doi.org/10.1189/jlb.5AB0915-406R
- Rajasuriar et al., 2010 R. Rajasuriar, et al.; Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation; J. Infect. Dis., 202 (8) (2010), pp. 1254–1264
- Regidor et al., 2011 D.L. Regidor, et al.; Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation; AIDS, 25 (3) (2011), pp. 303–314
- Rhönsholt et al., 2013 F. Rhönsholt, H. Ullum, T.L. Katzenstein, J. Gertoft, S.R. Ostrowski; Persistent inflammation and endothelial activation in HIV-1 patients after 12 years of antiretroviral therapy; PLoS One, 8 (6) (2013), p. e65182
- Robinson et al., 2003 L.A. Robinson, et al.; The chemokine CX3CL1 regulates NK cell activity in vivo; Cell. Immunol., 225 (2) (2003), pp. 122–130
- Rodger et al., 2009 A.J. Rodger, et al.; Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection; J. Infect. Dis., 200 (6) (2009), pp. 973–983
- Sauce et al., 2007 D. Sauce, et al.; PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status; AIDS, 21 (15) (2007), pp. 2005–2013
- Sereti et al., 2009 I. Sereti, et al.; IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection; Blood, 113 (25) (2009), pp. 6304–6314
- Sheth et al., 2008 P.M. Sheth, et al.; Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation; J. Infect. Dis., 197 (10) (2008), pp. 1394–1401
- Shikuma et al., 2011 C.M. Shikuma, et al.; Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals; AIDS Res. Hum. Retrovir., 27 (5) (2011), pp. 461–468
- Shoelson et al., 2006 S.E. Shoelson, J. Lee, A.B. Goldfine; Inflammation and insulin resistance; J. Clin. Invest., 116 (7) (2006), pp. 1793–1801
- Siao and Tsirka, 2002 C.J. Siao, S.E. Tsirka; Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II; J. Neurosci., 22 (9) (2002), pp. 3352–3358
- Stone et al., 2005 S.F. Stone, P. Price, M.A. French; Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy; HIV Med., 6 (4) (2005), pp. 278–283
- Tebas, 2008 P. Tebas; Insulin resistance and diabetes mellitus associated with antiretroviral use in HIV-infected patients: pathogenesis, prevention, and treatment options; J. Acquir. Immune Defic. Syndr., 49 (Suppl. 2) (2008), pp. S86–S92
- Tuaillon et al., 2009 E. Tuaillon, et al.; Close association of CD8 +/CD38 bright with HIV-1 replication and complex relationship with CD4 + T-cell count; Cytometry B Clin. Cytom., 76 (4) (2009), pp. 249–260
- van Vonderen et al., 2009 M.G. van Vonderen, et al.; Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy; J. Infect. Dis., 199 (8) (2009), pp. 1186–1194
- Vigano et al., 2014 S. Vigano, et al.; CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression; PLoS Pathog., 10 (9) (2014), p. e1004380
- Wallet et al., 2010 M.A. Wallet, et al.; Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy; AIDS, 24 (9) (2010), pp. 1281–1290
- Weyand et al., 2014 C.M. Weyand, Z. Yang, J.J. Goronzy; T-cell aging in rheumatoid arthritis; Curr. Opin. Rheumatol., 26 (1) (2014), pp. 93–100
- Yin et al., 1998 M.J. Yin, Y. Yamamoto, R.B. Gaynor; The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta; Nature, 396 (6706) (1998), pp. 77–80
- Younas et al., 2015 M. Younas, C. Psomas, J. Reynes, P. Corbeau; Immune activation in the course of HIV-1 infection: causes, phenotypes and persistence under therapy; HIV Med. (2015) http://dx.doi.org/10.1111/hiv.12310
- Zevin et al., 2016 A.S. Zevin, L. McKinnon, A. Burgener, N.R. Klatt; Microbial translocation and microbiome dysbiosis in HIV-associated immune activation; Curr. Opin. HIV AIDS, 11 (2) (2016), pp. 182–190
- Zidar et al., 2015 D.A. Zidar, et al.; Oxidized LDL levels are increased in HIV infection and may drive monocyte activation; J. Acquir. Immune Defic. Syndr., 69 (2) (2015), pp. 154–160
- Zonios et al., 2008 D.I. Zonios, et al.; Idiopathic CD4 + lymphocytopenia: natural history and prognostic factors; Blood, 112 (2) (2008), pp. 287–294
Document information
Published on 06/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?