(Created page with "==Keywords== Adverse drug reaction; Active cutaneous anaphylaxis; Anaphylaxis; Urticaria; Skin test Dear Editor, Sugammadex is a modified type of γ-cyclodextrin containing...") |
m (Scipediacontent moved page Draft Content 935749437 to Hotta et al 2016a) |
(No difference)
| |
Latest revision as of 14:07, 5 April 2017
Keywords
Adverse drug reaction; Active cutaneous anaphylaxis; Anaphylaxis; Urticaria; Skin test
Dear Editor,
Sugammadex is a modified type of γ-cyclodextrin containing 8 thiopropionate side chains. It is used for the safe and rapid reversal of neuromuscular block, such as rocuronium or vecronium.1 Although several cases were reported as intraoperative anaphylaxis caused by sugammadex, the causative epitopes have not fully understood yet. Here, we present a case of anaphylaxis caused by the γ-cyclodextrin in sugammadex.
A 65-year-old woman underwent an endoscopic left paranasal sinus operation for a left maxillary cyst. She had undergone surgery for ileus 15 years ago. She had no history of food, drug or other allergies and had not been exposed to sugammadex previously. At the end of the operation, 200 mg sugammadex were administered to reverse a rocuronium-induced neuromuscular block. Two minutes later, she developed hypotension (systolic arterial pressure: <60 mm Hg), and erythema over her whole body. After the administration of ephedrine and hydrocortisone sodium succinate, her symptoms improved. Drug-induced anaphylaxis was suspected.
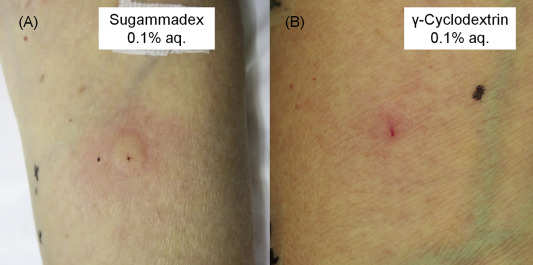
Two months later, we performed prick tests and intradermal tests based on the drugs and latex gloves used in the operation before the onset of anaphylaxis; i.e., we tested the effects of sugammadex (0.01, 0.1, 1, and 10% aq.), lidocaine hydrochloride, flurbiprofen axetil, fentanyl citrate, acetaminophen, propofol, and latex. The patients responses to the prick tests and intradermal tests were examined after 15 min and were graded according to the standard method reported by Dresborg et al.2 A response of grade 2 or greater that was stronger than the patients response to the negative control was considered to be positive. We also performed prick tests with saline as a negative control and histamine (10 mg/ml) as a positive control, and an intradermal test with saline as a negative control. The prick tests produced negative reactions for all agents. However, the intradermal test with 0.01% (aq.) sugammadex produced a grade 1 reaction, and that with 0.1% (aq.) sugammadex elicited a grade 2 reaction (an erythematous lesion measuring 28.3 × 32.8 mm and a wheal measuring 9.1 × 9.6 mm) (Fig. 1A). All of the other substances produced negative results. Anaphylaxis caused by sugammadex was subsequently diagnosed. Although the prick tests produced negative reactions for sugammadex, the intradermal test with it produced a positive reaction. That seemed to be because the dose of antigen absorbed into the skin was larger in the intradermal test than that in the prick test.
|
|
|
Fig. 1. Responses to the intradermal test of sugammadex (0.1% aq.) (A) and γ-cyclodextrin (0.1% aq.) (B) at 15 min. |
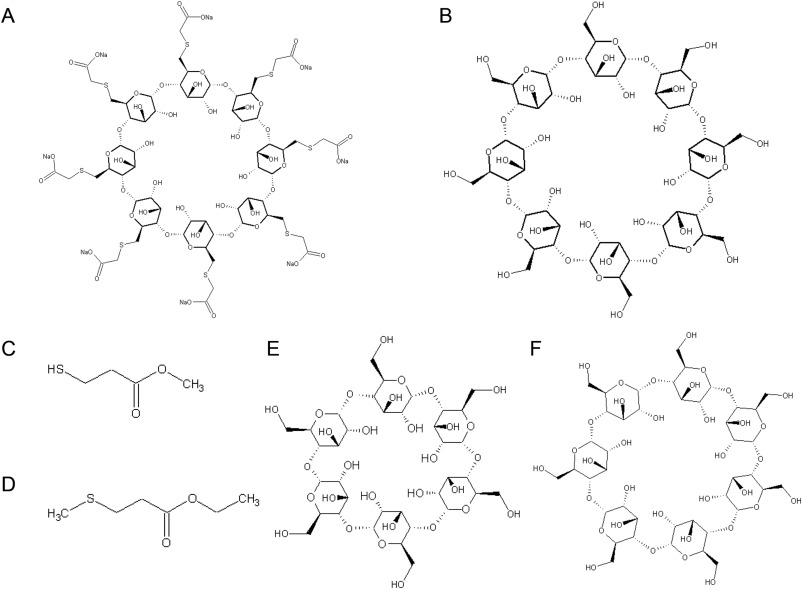
Sugammadex is a modified form of γ-cyclodextrin containing 8 thiopropionate side chains (Fig. 2A),1 so we then examined which epitope of sugammadex had induced the allergic reaction. To the best of our knowledge, the optimal concentrations of γ-cyclodextrin (Fig. 2B) for use during prick tests and intradermal tests have not been reported in the literature, so we performed the γ-cyclodextrin prick tests using the same concentration as were employed during the sugammadex prick tests (0.01, 0.1, 1, and 10% aq.), and the same approach was adopted for the intradermal tests of γ-cyclodextrin (0.001 aq. and 0.01% aq.; Wako, Japan). We also performed prick tests with methyl 3-mercaptopropionate (0.001% aq. and 0.01% aq.; Wako) (Fig. 2C) and ethyl 3-methylthiopropionate (0.001% pet. and 0.01% pet.; Wako) (Fig. 2D), which are found in thiopropionate. All of the prick tests produced negative reactions. The intradermal test of 0.01% (aq.) γ-cyclodextrin elicited a grade ± reaction, and that of 0.1% (aq.) γ-cyclodextrin produced a grade 2 reaction (an erythematous lesion measuring 24.7 × 25.7 mm and a wheal measuring 6.0 × 6.1 mm) (Fig. 1B). The intradermal test of saline produced negative results. Intradermal tests of γ-cyclodextrin (0.1% aq.) did not produce any reactions in three normal subjects. We did not perform intradermal tests with methyl 3-mercaptopropionate because it irritates the skin, or with ethyl 3-methylthiopropionate due to its insolubility in water. Based on these findings, anaphylaxis due to the γ-cyclodextrin in sugammadex was diagnosed. As γ-cyclodextrin contains 8 glucose units, and α- and β-cyclodextrin contain 6 and 7 glucose units, respectively (Fig. 2E, F),3 we also performed prick tests with α-cyclodextrin (10% aq.; Wako), β-cyclodextrin (10% pet.; Wako), and intradermal tests with α-cyclodextrin (0.01% aq. and 0.1% aq.). All of these tests produced negative reactions, indicating that the patient was sensitized with γ-cyclodextrin containing 8 glucose units. We did not carry out any intradermal tests with β-cyclodextrin due to its insolubility in water.
|
|
|
Fig. 2. Chemical structures of agents used in skin tests. The structures of sugammadex (A), γ-cyclodextrin (B), methyl 3-mercaptopropionate (C), ethyl 3-methylthiopropionate (D), α-cyclodextrin (E), and β-cyclodextrin (F) are shown. |
Several cases of intraoperative anaphylaxis after administration of sugammadex have been reported in the English literature.4, 5, 6, 7 and 8 Baldo et al.4 indicated that the use of carrier molecules such as cyclodextrins could change the immunological or allergic behavior of some encapsulated drugs; therefore, sugammadex-rocuronium complexes could become the potential allergen. In contrast, several cases 9 and 10 in which rocuronium-induced anaphylaxis was successfully managed with sugammadex (sugammadex encapsulates rocuronium molecules) have been reported. Sadleir et al.5 reported three cases of intraoperative anaphylaxis due to sugammadex. In two of three cases, the responses produced during skin test of a mixture of rocuronium and sugammadex were significantly attenuated compared with those elicited during skin tests involving sugammadex alone. Baldo et al.4 and Sadleir et al.5 proposed that skin tests of rocuronium, sugammadex, and rocuronium–sugammadex complex should be performed in patients that suffer allergic reactions after being given sugammadex to reverse neuromuscular block or rocuronium-induced anaphylaxis.
Neither our patient nor four previously reported patients who suffered sugammadex-associated hypersensitivity5 and 6 had previously been exposed to sugammadex. Tsur et al.7 reported that 12 cases out of 15 cases that suffered sugammadex-associated hypersensitivity had not previously been exposed to suggamadex. Cyclodextrins are used as a carrier and stabilizer for flavors, colors, fat-soluble vitamins, and polyunsaturated fatty acids.3 This suggests that the above patients might have become possibly sensitized to the cyclodextrins included in foods or daily goods although they had no history of allergy for foods containing cyclodextrins. To the best of our knowledge, this is the first report about anaphylaxis caused by the γ-cyclodextrin in suggamadex. Clinicians that use sugammadex should be aware that it is possible for patients to suffer γ-cyclodextrin-induced anaphylaxis.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1 M. Naguib; Sugammadex: another milestone in clinical neuromuscular pharmacology; Anesth Analg, 104 (2007), pp. 575–581
- 2 S. Dreborg, A. Backman, A. Basomba, J. Bousquet, P. Dieges, H.J. Malling; Skin tests used in type I allergy testing. Position paper prepared by the subcommittee on skin tests of the European Academy of Allergology and Clinical Immunology; Allergy, 44 (1989), pp. S22–S30
- 3 I.C. Munro, P.M. Newberne, V.R. Young, A. Bär; Safety assessment of gamma-cyclodextrin; Regul Toxicol Pharmacol, 39 (2004), pp. S3–S13
- 4 B.A. Baldo, N.J. McDonnell, N.H. Pham; Drug-specific cyclodextrins with emphasis on sugammadex, the neuromuscular blocker rocuronium and perioperative anaphylaxis: implications for drug allergy; Clin Exp Allergy, 41 (2011), pp. 1663–1678
- 5 P.H. Sadleir, T. Russell, R.C. Clarke, E. Maycock, P.R. Platt; Intraoperative anaphylaxis to sugammadex and a protocol for intradermal skin testing; Anaesth Intensive Care, 42 (2014), pp. 93–96
- 6 J. Jeyadoss, P. Kuruppu, N. Nanjappa, R. Van Wijk; Sugammadex hypersensitivity-a case of anaphylaxis; Anaesth Intensive Care, 42 (2014), pp. 89–92
- 7 A. Tsur, A. Kalansky; Hypersensitivity associated with sugammadex administration: a systematic review; Anaesthesia, 69 (2014), pp. 1251–1257
- 8 K. Godai, M. Hasegawa-Moriyama, T. Kuniyoshi, T. Kakoi, K. Ikoma, S. Isowaki, et al.; Three cases of suspected sugammadex-induced hypersensitivity reactions; Br J Anaesth, 109 (2012), pp. 216–218
- 9 N.J. McDonnell, T.J. Pavy, L.K. Green, P.R. Platt; Sugammadex in the management of rocuronium-induced anaphylaxis; Br J Anaesth, 106 (2011), pp. 199–201
- 10 T. Kawano, T. Tamura, M. Hamaguchi, T. Yatabe, K. Yamashita, M. Yokoyama; Successful management of rocuronium-induced anaphylactic reactions with sugammadex: a case report; J Clin Anesth, 24 (2012), pp. 62–64
Document information
Published on 05/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?