(Created page with "==Abstract== The upper Malwathu Oya is a seasonal river. The main livelihood of people living in the immediate vicinity of the river is paddy cultivation, and chronic kidney...") |
m (Scipediacontent moved page Draft Content 450864603 to Perera et al 2016a) |
(No difference)
| |
Latest revision as of 14:30, 27 March 2017
Abstract
The upper Malwathu Oya is a seasonal river. The main livelihood of people living in the immediate vicinity of the river is paddy cultivation, and chronic kidney disease is reported among them. Farmers utilize different types of agricultural chemicals in their fields expecting bumper harvests. Several agricultural chemicals have been reported to contain toxic trace elements in Sri Lanka. Therefore, arsenic and cadmium might end up in the river water. The presence of these trace elements in the river water and sediments can result in their bioaccumulation in fish tissues. The main purpose of this study was to investigate the presence of two trace elements in water and sediments, as well as in fish tissues (gills, kidney, liver and muscle) of three food fish species, Etroplus suratensis , Anabas testudineus and Channa striata during cultivating and non-cultivating seasons of the year. Further, the level of bioaccumulation of two trace elements in fish tissues in relation to the contamination level of water and sediments was assessed. Data were gathered for 43 months. Arsenic and cadmium concentration in water showed a significant (P < 0.05) seasonal variation. Generally, the two trace elements in the river water were highest during the cultivating seasons than in other seasons. In all species, both trace elements in the gills highly depended on the concentration in the water. In all species, two trace elements in water and sediment did not significantly affect the levels in muscle tissue. Therefore, the trace element levels in the edible parts of these three fish were well below the maximum permissible levels of international institutions.
Keywords
Arsenic ; Bioaccumulation ; Cadmium ; Fish ; Trace elements ; Water
Introduction
Arsenic (As) and cadmium (Cd) are widely found in the environment (Patrick, 2003 ) both from natural occurrence and due to anthropogenic activity (EFSA, 2009 ). Use of agricultural chemicals has been indicated as the main anthropogenic source of As and Cd pollution in aquatic environments of Sri Lanka (Illeperuma, 2000 ; Jauasumana et al ., 2011 ). As both trace elements are potentially toxic (Patrick, 2003 ; Godt et al ., 2006 ; Wang et al ., 2012 ; Chen et al ., 2015 ) at some bioavailability (Luoma and Rainbow, 2008 ), their presence in aquatic environments can result in deleterious effects on aquatic organisms (Mason et al., 2000 ). Similarly, As and Cd can exist in both abiotic (water and sediments) and biotic (organisms) components of aquatic environments at different concentrations. However, toxicity occurs in aquatic animals when the rate of uptake of a trace element exceeds the combined rates of efflux and detoxification of metal into metabolically inert forms (Luoma and Rainbow, 2005 ). In fish, metal uptake differs fundamentally from that of terrestrial animals as they are constantly submerged in the solution of metal ions (Perera et al., 2015 ). As a result, metal distribution in fish is determined mainly by its content in water and food (Farkas et al ., 2000 ; Mohamed, 2008 ). Further, fish have a tendency to accumulate heavy metals depending on their position in the food chain and their feeding habits (Houserová et al., 2007 ). Therefore, the concentration of As and Cd in water and bottom sediment, as well as the trophic position of fish can have significant effects on bioaccumulation of these two trace elements in fish.
Malwathu Oya (“Oya” refers to seasonal rivers) is the second longest (164 km) river which originates from a mountain range in the North Central Province (NCP) in Sri Lanka (The National Atlas of Sri Lanka, 2007 ). The upper reach of Malwathu Oya (latitude 8°15′ and 8°05′, longitude 80°26′ and 80°40′) runs across a remote area that consists of two distinct land use types, forest areas and paddy lands. People living in the upper Malwathu Oya area mainly depend on paddy cultivation as their livelihood (Perera et al., 2014 ). All paddy farmers in the area utilize different types of weedicides, pesticides, fungicides, rodenticides and inorganic fertilizers expecting high yield (Perera et al., 2014 ). The water utilized in their paddy lands diverts directly or indirectly to the upper Malwathu Oya by paddy outlet canals (POCs).
Several studies reported the prevalence of overusing agricultural chemicals by farmers in many parts of Sri Lanka (Selvarajah and Thiruchelvam, 2007 ; Padmajani et al ., 2014 ). NCP of Sri Lanka is famous for chronic kidney disease (CKDu1 ) (Kabata et al ., 2016 ; Wimalawansa, 2015a ; Wimalawansa, 2015b ) of which the apparent aetiology is most controversial among intellectuals. According to studies, As and Cd may be vital in spreading the disease among farmers in NCP (Jayasumana et al ., 2014a ; Jayasumana et al ., 2014b ). Many studies have been carried out in the NCP to investigate the impacts of inorganic fertilizers and other agricultural chemicals (e.g., pesticides and weedicides) that can contribute As and Cd into aquatic environment (Illeperuma, 2000 ; Jauasumana et al ., 2011 ; Kumar and Singh, 2010 ). Paddy cultivation activities that take place in the upper Malwathu Oya catchment would also release As and Cd into the water. As a result, As and Cd can appear in water, sediment and fish tissues. Accumulation of As and Cd in fish tissues can result in deleterious effects on fish (Govind and Madhuri, 2014 ). However, various metals show different affinities to fish tissues (Jezierska and Witeska, 2006a ; Jezierska and Witeska, 2006b ). For example, the non-edible tissues of fish can accumulate more metals than the edible muscle meat (Canli et al ., 1998 ; Jezierska and Witeska, 2006a ; Jezierska and Witeska, 2006b ; Mohamed, 2008 ; Ambedkar and Muniyan, 2011 ). As freshwater fish is the cheapest source of animal protein for villagers in the NCP (Amarasinghe and Silva, 1999 ), the elevated levels of As and Cd in edible fish tissues can cause health risks (EFSA, 2009 ) as well.
Therefore, the main the purpose of this study is to investigate the effects of paddy cultivating activities (which take place in nearby river catchments) on the river water and fish. To understand the effects, the current study mainly focuses on As and Cd in water and sediments, and as the two elements have created a platform of arguments in various aspects of CKDu, in three common food fish species, Etroplus suratensis , Anabas testudineus and Channa striata ( Pethiyagoda, 1991 ), collected from forest and paddy cultivating areas of upper Malwathu Oya. E. suratensis is mainly a herbivore ( Pethiyagoda, 1991 ) and habitual bottom feeder ( Costa, 1983 ; Joseph and Joseph, 1988 ; Vidhya and Radhakrishnan, 2012 ). A. testudineus is an omnivore ( Pethiyagoda, 1991 ; Zalina et al ., 2012 ) and C. striata is highly carnivorous ( Ali, 1990 ; Courtenay and Williams, 2004 ). As the trace element accumulating tendency in different fish species and their tissues can be varied, four tissues (gill, liver, kidney and muscle) of the above-mentioned three fish species were analysed for As and Cd. Further, this study assessed the level of accumulation of As and Cd in fish tissues in relation to the contamination level of water and sediments and tried to predict the suitability of these three fish species for use as bioindicators. Finally, the human health risk due to consumption of the muscle meat of Malwathu Oya fish will be discussed.
Materials and Methods
Study Location and Sampling Sites
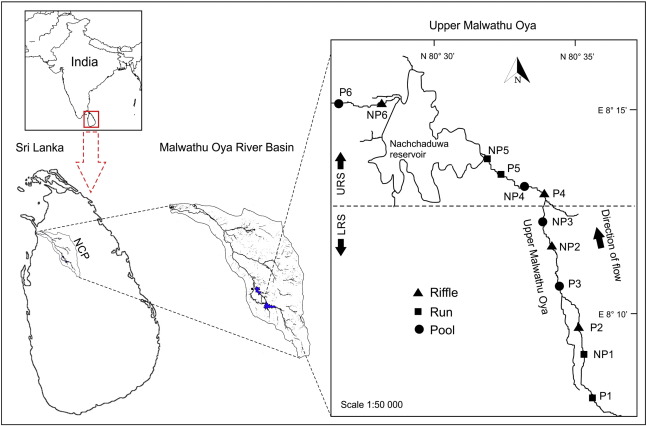
This study was conducted in a 30-km-long reach in upper Malwathu Oya between Ritigala Strict Nature Reserve (latitude 8°12′ and longitude 80°65′) and Nachchaduwa perennial reservoir (latitude 8°15′ longitude 80°29′) in the Anuradhapura District in the NCP of Sri Lanka (Fig. 1 ). The first 17 km stretch of the river was considered as the Upper River Segment (URS) and the second 13 km stretch was considered as the Lower River Segment (LRS). Twelve sampling sites were selected from the two different types of land uses (forest and paddy lands) of the river catchment. Six sampling sites were situated in forest areas (non-paddy) while the other six sites were situated in paddy cultivating areas.
|
|
|
Fig. 1. Map of the study area showing locations of sampling sites. |
Sample Collection, Preparation and Analysis
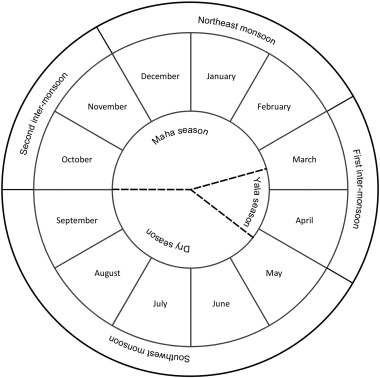
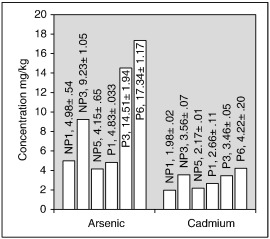
Water, sediment and fish samples were collected monthly from March 2012 to September 2015 (43 months). Water samples were collected from paddy-area-sites in the URS (P1–P3) and the LRS (P4–P6) as well as from forest-area-sites in the URS (NP1–NP3) and the LRS (NP4–NP6) in upper Malwathu Oya (Fig. 1 ) during all four monsoon seasons (first inter-monsoon, FIM; southwest monsoon, SWM; second inter-monsoon, SIM; and northeast monsoon, NEM) of the year (Fig. 2 ). Water samples from POCs were collected from six sampling sites (POC1–POC6) only during FIM, SIM and NEM as water was unavailable during the SWM season. Sediment and fish were collected from only six sampling sites from the paddy-area-sites (P1, P3, P6) and forest-area-sites (NP1, NP3 and NP5) because all three fish species selected for the study were not found in other study sites during some seasons of the year.
|
|
|
Fig. 2. Distribution of climatic seasons in the NCP. |
Water
High-density polyethylene (HDPE) bottles (500 mL) were used to collect water samples. Acid-washed (10% hydrochloric acid), sampling bottles were rinsed three times with distilled water to avoid any contamination from metal and non-metal ions. Each pre-washed bottle was rinsed again (three times) with the river water at each corresponding site before collecting water samples. In deep sites (non-wadeable), surface, middle and bottom layer samples were taken. Middle and bottom layer samples were collected using a Kemmerer-type water sampler (Stednick, 1991 ). Surface water samples were collected using sample bottles. The value of each parameter was calculated by the mean of the three layers. In moderately deep sites (wadeable), surface and bottom water samples were collected. The value of each parameter was calculated by the mean of two layers. In shallow sites, samples were collected from midway between the surface and the bottom (EPA, 1997 ). Water samples from six paddy outlet canals were collected from midway between the surface and the bottom using sample bottles about 10–15 m before the discharge points to assure that both water samples did not mix with each other. All water samples were collected at three different places in each study site. Duplicate water samples were collected at the same place, at the same time (EPA, 1997 ). The value of each parameter was calculated by the mean. In order to increase the precision of the results, all the samples were collected under clear weather conditions.
Water samples collected (to analyse total dissolved As and Cd) were preserved at 4 °C in ice and immediately transported to the laboratory. At the laboratory, all samples were filtered through 0.45 μm, 30 mm diameter PTFE syringe filters (Sigma-Aldrich, USA) to remove any particulates. Subsamples (lab replicates) of 100 mL filtered water samples were acidified to pH < 2 (verified with pH test strips, HACH, USA), labelled and kept at 4 °C in the refrigerator until further analysis (APHA, 2005 ).
Sediment
Sediments of non-wadeable sites were collected from the top 10 cm of surface with a benthic grab sampler (Ekman Grab Kit, Hoskin Scientific, Canada) and placed into a 500 mL glass jar. Sediment samples from wadeable areas were collected into polyethylene bags using a Teflon scoop. Sediment samples were transported to the laboratory immediately and freeze-dried before analysis (Cheung et al., 2003 ). Sample containers and tools were cleaned as described by quality assurance and quality control specifications from the EPA (1990) . In the laboratory, samples were dried at 65 °C for 24 h and ground. Powdered sediment samples were then passed through a 63 μm mesh sieve to separate large fractions (Alhashemi et al., 2012 ). The sieved samples were labelled and prepared for analysis.
Fish
Five to eight samples from E. suratensis , A. testudineus and C. striata were collected each month from local fishers in upper Malwathu Oya. The length and weight of each fish was recorded at study sites ( Table 1 ). Adult specimens of E. suratensis (n = 246), A. testudineus (n = 322) and C. striata (n = 218) in similar lengths were collected. Fish samples were packed in polythene bags and transported to the laboratory in an insulated box with crushed ice.
| Sampling site | E. suratensis | A. testudineus | C. striata | |||
|---|---|---|---|---|---|---|
| Total length (cm) | Weight (g) | Total length (cm) | Weight (g) | Total length (cm) | Weight (g) | |
| P1 | 21.3 ± .53 | 255 ± 9.1 | 19.4 ± .38 | 78 ± 4.4 | 40.4 ± .55 | 625 ± 13.1 |
| P3 | 21.5 ± .41 | 262 ± 8.6 | 18.7 ± .33 | 72 ± 3.5 | 40.8 ± .47 | 642 ± 12.0 |
| P6 | 21.2 ± .50 | 247 ± 8.1 | 18.9 ± .41 | 75 ± 3.8 | 41.3 ± .52 | 659 ± 12.6 |
| NP1 | 20.2 ± .46 | 241 ± 7.9 | 19.0 ± .30 | 78 ± 4.2 | 41.6 ± .43 | 655 ± 11.5 |
| NP3 | 21.5 ± .44 | 260 ± 4.5 | 19.5 ± .45 | 81 ± 4.0 | 40.9 ± .45 | 634 ± 14.7 |
| NP5 | 20.6 ± .38 | 242 ± 9.8 | 18.5 ± .24 | 69 ± 3.1 | 41.7 ± .50 | 647 ± 13.5 |
Specimens were descaled and washed thoroughly (Eneji et al., 2011 ). Dissections were performed on a clean bench using a titanium knife and Teflon forceps as described by Liao et al. (2003) . The different organs (gill, kidney, liver and muscle) were carefully dissected after rinsing with double-distilled water (Akan et al., 2009 ). Due to difficulties in obtaining sufficient amounts of tissue for analysis, adequate portions of the liver and kidney from several fish specimens were collected and these samples were pooled as described by (Leeves, 2011 ). Each pooled sample consisted of kidney and liver tissues of five individuals of approximately similar in length (± 0.5 cm). Muscle and gill tissues were not pooled as adequate portions were available for chemical analysis. The dissected tissues samples were placed in labelled clean polyethylene bags and immediately deep-frozen at − 20 °C until prepared for analysis (Taweel et al., 2011 ).
Chemical Analysis
For chemical analysis, water, sediment and tissue samples were transported to the Analytical Chemistry Laboratory, National Aquatic Resources Research Development Agency (NARA, Sri Lanka) in an insulated box with crushed ice during night time. Tissue samples were prepared for analysis as described by Jinadasa et al. (2013) . Tissues from three fish species were oven-dried at 105 °C. These dried samples were powdered and preserved in airtight polythene bags until further analysis. Tissue and sediment samples were digested using CEM XP-1500 (CEM, Matthews, USA) microwave accelerated system. Around 0.5 g of an oven-dried fish sample and powdered sediment samples were weighed accurately to four decimal places in a Teflon vessel. A total of 10 mL of 65% conc. HNO3 (AR, Sigma) was added and allowed to stand for 15 min in a fume hood for pre-digestion. Then, the Teflon vessel was connected to a microwave digester and digestion was carried out (at 200 °C for 25 min). The digested tissue and sediment samples were transferred to 50 mL volumetric flasks and made up to the mark with deionized water.
A total of 100 mL from each water sample was transferred into Pyrex beakers containing 10 mL of conc. nitric acid. The samples were boiled slowly and then evaporated on a hot plate to the lowest possible volume (about 15 mL). The beakers were allowed to cool and another 5 mL of conc. nitric acid was added. Heating was continued with the addition of conc. nitric acid as necessary until digestion was complete. The samples were evaporated again to dryness and the beakers were allowed to come to room temperature followed by the addition of 5 mL of HCl solution (1:1 v /v ). The solutions were warmed and 5 mL of 5 M NaOH was added, and then filtered. The filtrates were transferred to 100 mL volumetric flasks and diluted to the mark with distilled water. These solutions were then used for the elemental analysis.
Two reagent blank samples and two spiked samples were prepared for fish, sediment and water samples using the same method. The spiked samples were routinely analysed as the method of validation procedure. The recovery limits were maintained between 75% and 125% during the analysis of all sets of samples. The limit of detection (LOD; mean blank + 3S) was calculated for all metals and results of less than LOD were considered as not detected by the instrument.
Two trace elements were determined using an atomic absorption spectrophotometer (Varian240 FS, Varian Inc., Walnut Creek, CA, USA). A graphite tube atomizer (Varian GTA-120) was used for Cd determination and a vapour generation accessory (Varian VGA 77) with open-end cell was used for As determination. The calibration curves for the absorption two trace elements were performed with a standard solution of particular elements at optimum wavelength. Subsequent to the calibration, the reagent blank samples, unknown samples and spiked samples were aspirated into the atomic absorption spectrophotometer and results were recorded.
Data Analysis
All data were analysed using SAS statistical software (SAS version 9.0). In the first analysis, factorial experiments were used to study interactions between different factors. Land uses (paddy area and forest area), climate seasons (FIM, SWM, SIM and NEM), channel geomorphic units (CGUs; pool, riffle and run) and river segments (URS and LRS) were considered as factors. For each treatment combination, three replicates were taken. To study As and Cd in abiotic components (water samples), in the SAS procedure, a general linear model was used due to missing data during the drought season (SWM).
In the second analysis, a factorial experiment was used to study interaction between factors. Seasons (FIM, SWM, SIM and NEM), species (E. suratensis , A. testudineus and C. striata ) and tissues (gill, kidney, liver and muscle) were considered as factors and for each treatment combination, three replicates were taken. To study As and Cd in biotic components (tissues), in the SAS procedure, proc. ANOVA was used. A smaller number of fish tissues (4) were considered in this study which resulted in a smaller number of treatment comparisons. Therefore, LSD was used.
Data of arsenic and cadmium in water and fish tissues were tested for normality. Arsenic and cadmium levels in water showed p values of 0.159 and 0.291, respectively. Tissue arsenic and cadmium levels showed p values of 0.262 and 0.181, respectively. Therefore, all data had distributed normally.
Thirdly, the correlation (Pearson) between trace metal content in water of each study site and each tissue of three fish species found in corresponding study sites was studied. Similarly, the correlation (Pearson) between trace metal content in sediment samples of each study site and each tissue of three fish species found in corresponding study sites was studied. Correlation type (positive or negative) and significant levels were generated. All graphs were generated using MS Excel 2013 software.
Results and Discussion
Arsenic and Cadmium in Abiotic Components
Paddy Outlet Canals
Arsenic concentrations in POCs ranged annually from 111 to 498 μg/L and Cd concentrations ranged from 76 to 245 μg/L (Table 2 ). The concentration of both As and Cd in POCs was higher than the corresponding study sites in upper Malwathu Oya (Table 3 ; Table 4 ). Mean As and Cd concentration in POCs significantly (p < 0.05) changed in three seasons associated with paddy cultivation. In the majority of POCs, the concentration of both As and Cd was highest during FIM and it was always lowest during NEM. Arsenic concentrations varied in different seasons as FIM > SIM > NEM except in POC2 and POC3 where the concentration was highest during SIM. Similarly, the Cd concentration varied as FIM > SIM > NEM except in POC2 where the concentration between FIM and SIM was not significantly different (p > 0.05).
| Climatic season | |||
|---|---|---|---|
| FIM | SIM | NEM | |
| Arsenic | |||
| POC1 | 433.2a | 385.0b | 142.0c |
| POC2 | 344.4a | 352.0a | 148.3b |
| POC3 | 243.0b | 364.2a | 140.4c |
| POC4 | 473.2a | 459.4b | 130.2c |
| POC5 | 498.2a | 277.3b | 154.2c |
| POC6 | 465.1a | 231.1b | 111.0c |
| Cadmium | |||
| POC1 | 148.5a | 126.2b | 094.0c |
| POC2 | 142.0a | 143.4a | 076.1b |
| POC3 | 243.0a | 220.1b | 151.2c |
| POC4 | 213.2a | 184.1b | 170.1c |
| POC5 | 245.1a | 192.4b | 148.2c |
| POC6 | 145.1a | 131.0b | 147.4a |
Values within rows are significantly different at p < 0.05.
| Land use | Segment | Site | CGU | FIM | SWM | SIM | NEM |
|---|---|---|---|---|---|---|---|
| Paddy area | URS | P1 | Run | 43.3a | 09.1c | 32.0b | 03.1d |
| P2 | Riffle | 34.0a | 04.0b | 35.2a | 05.3b | ||
| P3 | Pool | 21.0a | 18.1a | 20.3a | 04.2b | ||
| LRS | P4 | Riffle | 46.3a | 05.3c | 37.4b | 09.1c | |
| P5 | Run | 48.1a | † | 41.5a | 11.3b | ||
| P6 | Pool | 15.3a | 09.4b | 19.0a | 02.0c | ||
| Forest area | URS | NP1 | Run | 14.0a | 01.1c | 10.3b | 01.1c |
| NP2 | Riffle | 09.0a | † | 01.3b | 01.1b | ||
| NP3 | Pool | 01.1c | 08.2a | 04.4b | 01.1c | ||
| LRS | NP4 | Pool | 01.4b | 03.0b | 09.1a | 01.3b | |
| NP5 | Run | 20.2a | 07.0b | 19.3a | 01.1c | ||
| NP6 | Riffle | 15.3a | 09.4b | 17.2a | 01.1c |
a, b, c, d are based on LSMEAN (adjusted mean) separation procedure. Values within rows having different superscripts are significantly different (P < 0.05). † , Not measured due to unavailability of water.
| Land use | Segment | Site | CGU | FIM | SWM | SIM | NEM |
|---|---|---|---|---|---|---|---|
| Paddy area | URS | P1 | Run | 94.2a | 14.0d | 54.2b | 34.4c |
| P2 | Riffle | 78.3a | 17.5d | 58.1b | 38.5c | ||
| P3 | Pool | 53.1a | 09.3c | 49.2a | 31.0b | ||
| LRS | P4 | Riffle | 110.2a | 09.4d | 68.3b | 44.0c | |
| P5 | Run | 134.0a | † | 92.4b | 43.2c | ||
| P6 | Pool | 80.1a | 03.2d | 45.2b | 25.0c | ||
| Forest area | URS | NP1 | Run | 35.1a | 02.5c | 27.0b | 01.1c |
| NP2 | Riffle | 24.0b | † | 26.3a | 01.1c | ||
| NP3 | Pool | 11.2a | 01.2c | 09.0b | 02.5c | ||
| LRS | NP4 | Pool | 25.0a | 03.0c | 18.3b | 02.3c | |
| NP5 | Run | 42.2a | 19.0c | 33.4b | 13.4c | ||
| NP6 | Riffle | 46.1a | 08.1d | 30.2b | 10.4c |
a, b, c, d are based on LSMEAN (adjusted mean) separation procedure. Values within rows having different superscripts are significantly different (P < 0.05). † , Not measured due to unavailability of water.
Paddy outlet canals are small waterways constructed by farmer organizations to drain water utilized from paddy lands to the upper Malwathu Oya. Any type of application that takes place in the paddy lands affects POCs as they are directly connected to the paddy lands. For example, inorganic fertilizers and other types of chemicals applied onto paddy lands get mixed with water and drain into POCs.
In Sri Lanka, it is generally accepted that four distinct climatic seasons (Fig. 2 ) can be identified during a twelve month period (Punyawardena, 2004 ; The National Atlas of Sri Lanka, 2007 ). Therefore, the rainfall seasons range from March of the current year to February of the following year (Department of Meteorology, 2014 ). Even if monsoonal rains account for a major share of the annual rainfall, they do not bring about homogeneous rainfall regimes over the whole island (Department of Agriculture, 2006 ). Therefore, out of these four monsoon seasons, two consecutive rainy seasons, SIM and NEM, make up the major growing season (Maha season) while the minor cultivating season (Yala season) is limited to the FIM and first part of the SWM (Fig. 2 ). Therefore, the period between these two seasons (from mid-May to September) is rather dry.
In the upper Malwathu Oya area (which belongs to the NCP), the majority of farmers engage in tillage activities with the onset of the SIM (in Maha) than FIM (in Yala) because the extended rainfall regimes during Maha season allow farmers to cultivate more paddy lands. However, the observed concentration of As and Cd was lower during the SIM than the FIM due to dilution effects of chemical compounds as an effect of high-intensity and extended rainfall in the Maha season. Nevertheless, As and Cd distribution in POCs show a binomial distribution related to two major cultivating seasons in the study area. As rain continues during the SIM and NEM seasons without a distinct interval (Fig. 2 ), further dilution of trace elements in the POCs is quite possible. As a result, the lowest concentrations were detected during the NEM.
However, the concentration of trace elements in POCs depends on many factors. For example, application frequency and strength can determine how much will be recovered in the POCs. Farmers (in the NCP) apply liquid and solid agro-chemicals into paddy lands in excessive quantities and in adequate frequencies (more than recommended times) expecting bumper harvests (Wimalawansa, 2015a ; Wimalawansa, 2015b ). A similar situation was observed in the upper Malwathu Oya area as well. As a result of these indiscriminate applications, the surplus will be recovered in the paddy-land-soil and finally wash off into POCs. As POCs are small waterways, changes in application frequencies and quantities of agricultural chemicals in paddy lands would result in obvious changes in their water chemistry. As a result, the distribution of As in some POCs (e.g., As in POC2 and POC3 is higher during SIM) is different from the regular fluctuation pattern.
Inappropriate use of agricultural chemicals is common in Sri Lanka. For example, Padmajani et al. (2014) observed that 53% of farmers in vegetable cultivating areas in the upcountry mix two or more chemicals together to make a cocktail mixture while 71% of them repeatedly apply the chemical to the same crop as they believe that the recommendations and prescriptions given in the pesticide product labels are insufficient. Selvarajah and Thiruchelvam (2007) described that about 60% paddy and chilli farmers apply 30%–40% higher concentrations of agricultural chemicals in Vavuniya District in Sri Lanka. These examples stress that the quantity and frequency of application of agricultural chemicals in Sri Lanka mostly depend on the individual preference of farmers. Therefore, chemical concentration in POCs reluctant to change according to the application practises of farmers. Furthermore, Paranagama (2013) states that analysis of As content in Sri Lankan soils, particularly in agricultural areas, gradually decreases with depth, implying that it is not present naturally in soils; nevertheless, it has been introduced from the surface, most probably due to anthropogenic activities. Paranagama (2013) reported that the highest total As content was reported from imported triple super phosphate used for rice cultivation, which ranged from 25.49 mg/kg to 37.86 mg/kg in CKDu endemic areas of NCP. Similarly, Jauasumana et al. (2011) indicated that 29 out of 31 pesticide brands widely used in Sri Lanka contain As in significant amounts ranging from 180 ± 14 μg/kg (in imidacloprid collected from Colombo) to 2586 ± 58 μg/kg (in glyphosate collected from Anuradhapura) per kg of pesticide. Wijesundara et al. (2013) observed that similar to nitrogen and phosphates, Cd concentration in reservoir water in the dry zone was elevated in the months of April and May in the Yala season, while relatively lower levels were recorded for the Maha season. They also reported that even if the other major nutrients (nitrogen, phosphorous and potassium) are added as chemical fertilizers, Cd is not added for any field as it has no any beneficial effect on crop life. Dissanayake and Chandrajith (2009) reported that phosphate fertilizers are produced by the acidulation of crushed and powdered phosphate rock. Therefore, contamination of phosphate fertilizer by toxic elements can be significantly high in some final fertilizer products (Dissanayake and Chandrajith, 2009 ).
Since As and Cd compounds are contained in agricultural chemicals, they will be recovered in POCs. Moreover, the distinct seasonal variability of As and Cd in POCs parallel to the major paddy cultivating seasons stresses that the origin of trace elements are rather agriculture-based. Improper cultivating practises of farmers seem to intensify the trace element levels in POCs. As POCs drain into the upper Malwathu Oya, assessment of the impacts on it is essentially important.
Upper Malwathu Oya
Seasonal Variation
Since the higher order interaction was significant (p < 0.0001), keeping three factors (land use, CGU and river segment) constant, compared the As and Cd content between seasons. Results of the mean separation revealed that As and Cd concentrations in the upper Malwathu Oya showed a distinct seasonal variation. As concentrations in sampled waters ranged from 1 to 48 μg/L (Table 3 ) and Cd concentrations ranged from 1 to 134 μg/L (Table 4 ). Both As and Cd concentrations were higher in paddy-area-sites than forest-area-sites. Concentrations of both trace elements were higher during FIM and SIM than SWM and NEM. Mean As concentration was significantly different (p < 0.05) between FIM and SIM in many sites (P1, P4, NP1, NP2, NP3 and NP4). Cadmium concentration was significantly higher (p < 0.05) during FIM than SIM in many sites (except in P3 and NP2). The lowest As and Cd values were detected during NEM and SWM seasons, respectively. Further, the mean As and Cd concentration in all sampling sites during FIM and SIM were higher than the recommended values (As, 0.01 mg/L (10 μg/L); Cd, 0.003 mg/L (3 μg/L)) in drinking water (WHO, 2006 ).
As observed in POCs (Paddy Outlet Canals ), distinct variation in two trace elements (Table 3 ; Table 4 ) in the river water is mainly associated with the paddy cultivating seasons. For example, relatively higher As and Cd concentrations observed in paddy-sites (than those in forest-sites) indicate that POCs contribute As and Cd from paddy lands. The higher As and Cd concentrations during FIM and SIM is related to Yala and Maha season cultivating activities which results water quality changes due to the contribution of polluted water from POCs. The distinct difference between trace element concentrations between FIM and SIM is associated with the similar reasons discussed under POCs in Paddy Outlet Canals . However, unlike the observations in POCs, many other factors such as river hydrology (e.g., flow rate and river discharge), nature of channel geomorphology (e.g., riffles, runs and pools) and other water quality parameters can also alter the trace element concentrations (Meybeck et al ., 1992 ; Zielinski et al ., 2003 ). Hydrological changes are mainly seasonal due to rainfall regimes during the wet season and due to higher evaporation during the dry season (Fig. 2 ). Extended high-intensity rainfall associated with Maha season determines the lowest trace element concentration during the NEM. Lack of inflow from POCs results in the lowest trace element concentration during SWM. The behaviour of Cd is more rhythmic during the two inter-monsoon seasons. Nevertheless, the behaviour of As is more irregular between study sites. The highest mean concentrations of As and Cd in study sites during FIM is related to the lower dilution of trace elements due to the less extended Yala season rains. Nevertheless, insignificant concentrations in P3 and relatively low concentration during FIM in NP2 stress the complex behaviour of Cd in water. However, the behaviour of As is more complicated than Cd. For example, in six study sites (exactly half of study sites), the irregular behaviour of As stresses that other than the application strength and quantities of agrochemicals many other factors are involved in the determination of concentration in river water. Interactions between chemical, hydrological and geomorphological parameters in water can alter the general seasonal rhythmic pattern of trace elements between FIM and SIM. However, the relationship between POCs and the river water with respect to the two trace elements is useful to understand the behaviour of As and Cd more closely in study sites during the FIM and SIM seasons ( ).
Correlation between POCs and River Sites
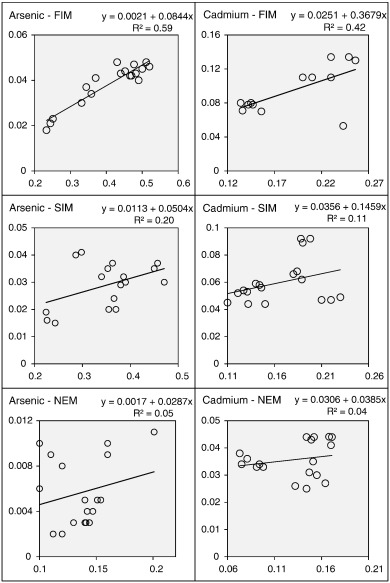
If paddy cultivating activities affected the river water quality, the concentration of As and Cd in paddy-sites (P1–P6) should increase according to the concentration of those trace elements in corresponding POCs. However, the strength of the above relationships can vary seasonally according to the nature of the cultivating activities and rainfall regimes. For example, As (r2 = 0.59, p = .001) and Cd (r2 = 0.42, p = .01) levels between POCs and paddy-sites are highly correlated during the FIM (Fig. 3 ). Nevertheless, it is poorly correlated during SIM (r2 = 0.20, p = .07 and r2 = 0.11, p = .17 for As and Cd, respectively) and NEM (r2 = 0.05, p = .34 and r2 = 0.04, p = .41 for As and Cd, respectively).
|
|
|
Fig. 3. Relationship between trace metal levels (mg/L) in POCs (X axis) and paddy-sites (Y axis). |
Pollutants that enter the flowing waters travel some distance before they are thoroughly mixed throughout the flow (EPA, 1997 ). The same phenomenon was observed with respect to trace element pollution in upper Malwathu Oya as well. Less extended and low-intensity rainfall regimes during the FIM do not encourage the mixing process as it does during the SIM and NEM (more extended and high-intensity rainfall) seasons. Therefore, the chemicals that brings by POCs can be recovered at relatively higher concentrations at the study sites (P1–P6). However, the strength of As and Cd that diverts through POCs can be weakened more easily due to relatively higher flow rates and river discharge during SIM and NEM. Further, the chemical concentration is relatively higher in POCs during the FIM than in the SIM and NEM (Table 2 ). Therefore, relatively higher contributions of trace elements from POCs and less efficient mixing process determines the strong correlation between POCS and river water.
Studies related to the seasonal trace element variations in aquatic environments in the NCP of Sri Lanka are rare. Many studies basically address the levels of As and Cd in different reservoirs without continuous assessments. However, according to Wijesundara et al. (2013) , Cd concentration in tank water (in a tank cascade close to upper Malwathu Oya) was elevated from April to May in the Yala season while relatively lower concentrations were recorded during the Maha season. This result is similar to the observations of the current study.
Arsenic and Cadmium in Biotic Components
Arsenic level in four tissues of each species varied as A. testudineus > C. striata > E. suratensis and Cd level varied as C. striata > A. testudineus > E. suratensis ( Fig. 4 ). Since the three-way interaction between seasons, species and tissues is significant (p < 0.0001), keeping two factors (season and species) constant, As (Table 5 ) and Cd (Table 6 ) between tissues were compared. As level between tissues is significantly different (p < 0.05) in all seasons. As accumulation pattern in tissues is similar in all fish in all seasons (kidney > liver > gill > muscle) (Table 5 ). Nevertheless, similar tissue level Cd accumulation patterns were observed only in A. testudineus and C. striata (liver > gill > kidney > muscle); it is different and irregular in E. suratensis ( Table 6 ).
|
|
|
Fig. 4. Arsenic and cadmum level in three fish species; ES, E. suratensis ; AT, A. testudineus ; CS, C. striata. |
| Species | Tissue | FIM | SWM | SIM | NEM |
|---|---|---|---|---|---|
| E. suratensis | Gill | 0.462c | 0.364c | 0.406c | 0.357c |
| Kidney | 3.438a | 3.080a | 3.213a | 3.155a | |
| Liver | 2.670b | 2.735b | 2.675b | 2.590b | |
| Muscle | 0.096d | 0.027d | 0.034d | 0.026d | |
| A. testudineus | Gill | 1.389c | 1.232c | 1.340c | 1.286c |
| Kidney | 4.223a | 4.116a | 4.732a | 4.490a | |
| Liver | 2.193b | 2.233b | 1.946b | 1.919b | |
| Muscle | 0.082d | 0.067d | 0.064d | 0.082d | |
| C. striata | Gill | 1.213c | 1.082c | 1.157c | 1.154c |
| Kidney | 4.184a | 4.321a | 4.071a | 4.303a | |
| Liver | 2.186b | 1.979b | 2.142b | 2.035b | |
| Muscle | 0.102d | 0.099d | 0.073d | 0.072d |
Values within columns are significantly different at p < 0.05 (mean comparison made column-wise for each species).
| Species | Tissue | FIM | SWM | SIM | NEM |
|---|---|---|---|---|---|
| E. suratensis | Gill | 0.056c | 0.034b | 0.080b | 0.030b |
| Kidney | 0.085a | 0.064a | 0.110a | 0.080a | |
| Liver | 0.075b | 0.065a | 0.087b | 0.081a | |
| Muscle | 0.001d | 0.001c | 0.001c | 0.001c | |
| A. testudineus | Gill | 0.327b | 0.344a | 0.347b | 0.307b |
| Kidney | 0.311c | 0.298b | 0.314c | 0.271c | |
| Liver | 0.395a | 0.340a | 0.386a | 0.360a | |
| Muscle | 0.004d | 0.022c | 0.018d | 0.018d | |
| C. striata | Gill | 0.386b | 0.486b | 0.459b | 0.374b |
| Kidney | 0.249c | 0.265c | 0.258c | 0.240c | |
| Liver | 0.521a | 0.500a | 0.527a | 0.536a | |
| Muscle | 0.005d | 0.025d | 0.013d | 0.015d |
Values within columns are significantly different at p < 0.05 (mean comparison made column-wise for each species).
Species Level Arsenic and Cadmium Accumulation
E. suratensis is omnivorous but mainly depend on plant matter ( Pethiyagoda, 1991 ). It feeds mainly on a wide variety of plant matter (from unicellular algae to macrophytes), dead organic matter and some animal matter (fish eggs, copepod, fish scales) (Vidhya and Nair, 2012 ). A. testudineus is an omnivore ( Pethiyagoda, 1991 ; Zalina et al ., 2012 ) and C. striata is highly carnivorous ( Ali, 1990 ; Courtenay and Williams, 2004 ). If the feeding habits altered As bioaccumulation, a variation in species level accumulation might be observed. Nevertheless, similar As accumulation tendencies in the tissues of the three species stresses that the affinity of fish towards arsenic does not change according to the feeding habits of fish. However, the similar affinities showed towards Cd by A. testudineus and C . striata , but not by E. suratensis , stresses that their feeding habits and habitat preferences affect bioaccumulation. As an air-breathing fish, C . striata prefers stagnant, slow-running and shallow water ( Mat Jais, 2007 ) while A. testudineus is mainly associated with turbid, stagnant habitats ( Pethiyagoda, 1991 ). C. striata mainly feed on fish and crustaceans while A. testudineus prefers macrophytes with shrimp and fish fry ( Pethiyagoda, 1991 ). Although the trophic positions of these two species are different, their habitat and feeding preferences (interest towards animal matter) are more or less similar. However, the seasonal changes in food availability, disparity in feeding habits and habitat preferences (benthopelagic) of E . suratensis might change the accumulation pattern of Cd in them.
Tissue Level Arsenic and Cadmium Accumulation
Trace elements (e.g., As, Cd) unlike organic pollutants, cannot be chemically degraded (Hooda, 2010 ) or biodegraded by microorganisms (Mermut et al., 1996 ). Therefore, these chemicals accumulate in living tissues any time they are taken up (Edem et al., 2008 ) and are stored faster than they are broken down (metabolized) or excreted (Extension Toxicology Network, 1993 ). As a consequence, bioaccumulation can develop at any concentration without minimum level requirements (Amiard et al ., 1987 ; Marcovecchio, 2004 ), and the capacity of bioaccumulation can be different according to the target tissues (Alhashemi et al ., 2012 ; El-Moselhy et al ., 2014 ).
Results indicate (Table 5 ) that the accumulation tendency of As in different tissues does not change according to the concentration in the medium. Therefore, in all fish species during all seasons (at different concentrations in water), arsenic level changed as kidney < liver < gill < muscle. However, the behaviour of Cd was different; the tissue level accumulation tendency in A. testudineus and C. striata was liver < gill < kidney < muscle in all seasons except in A . testudineus during SWM ( Table 6 ). The observed difference in A. testudineus is mainly associated with its response to dry weather conditions. Upper Malwathu Oya is a seasonal river, thus, during the dry season, in the latter part of the SWM, many areas of the river tend to dry up leaving water in pools and some runs. Because of their unique air-breathing ability, A. testudineus prefer to burrow into the mud to survive ( Pethiyagoda, 1991 ). This adaption might allow more contact with sediment and mud than other species, which can result in more Cd in their gills. On the other hand, the second highest level of Cd in the gill of A . testudineus and C. striata during other seasons (FIM, SIM and NEM) is mainly associated with its habitual bottom access as a feeding habit. For example, during feeding times, they access benthic animals which results in agitation of bottom sediment. Sediments contain higher content of Cd ( Fig. 5 ). Bioturbation (the mechanical disturbance of benthic sediments) by benthic organisms disrupts the water–sediment interface (Sandnes et al., 2000 ) and brings oxygen into the sediment, thus enabling Cd to re-dissolve (Delmotte et al., 2007 ). Repeating the same process by bottom-living fish tends to bathe their gills with Cd-rich water, which results in more Cd in the gill tissue. As benthopelagics, similar results were observed in E. suratensis as well. However, their accumulation pattern is different during the FIM ( Table 6 ); therefore, they might show different feeding habits; accessing more macrophytes and algae due to the availability of more plant matter during the FIM.
|
|
|
Fig. 5. Arsenic and cadmium levels in sediments. |
Fish uptake As (McIntyre and Linton, 2011 ) and Cd (Kamunde and MacPhail, 2011 ) directly from exposure to contaminated water or indirectly by consumption of food containing the chemical (Drexler et al., 2003 ). The difference in the levels of accumulation in different organs or tissues of fish is primarily attributed to the various physiological roles carried out in each organ (Kotze, 1997 ; Senthil et al ., 2004 ). Canli and Atli (2003) described that the fishs behaviour and feeding preferences along with some abiotic factors determine the bioaccumulation of trace elements in different tissues. Further, the accumulation of As and Cd in fish tissues strongly reflect the exposure pathways. For example, Szebedinszky et al. (2001) and Franklin et al. (2005) explained that in diet-exposed fish, Cd concentration is generally higher in the gastrointestinal tract than in the gills, whereas in the case of waterborne exposure, Cd concentration is generally higher in gills and kidneys. Similarly, the gills are the prime site for arsenic accumulation and reflects the excretory roles of the organs (Oladimeji et al., 1984 ). Moreover, the external position of the gills in fish and its well-branched structural organization, which results in a large volume of water passing through the gill surface, make the gills a prime site for trace element accumulation (Subathra and Karuppasamy, 2007 ). As explained by Szebedinszky et al. (2001) , more Cd may enter through the dietary paths which results in high Cd levels in the liver in all the fish species. Therefore, the order of arsenic accumulation kidney > liver > gill > muscle stresses that a waterborne pathway is more important in As bioaccumulation while the order liver > kidney > gill > muscle reflects the importance of dietary pathways in Cd bioaccumulation.
The highest arsenic level found (than other species) in the liver of E. suratensis and an irregular accumulation pattern was observed between A. testudineus and C . striata , which implies that the accumulation tendency does not increase with the trophic position of fish. If the arsenic was concentrated along the food chains, the relevant levels should be high at higher trophic positions. However, the highest level of As at lower trophic positions (E. suratensis ) compared to the higher trophic positions (represented by A. testudineus and C. striata ) indicated that although bioaccumulation occurs, there is no indication of biomagnification of arsenic along the aquatic food chains in upper Malwathu Oya. Therefore, As pollution is more severe in lower trophic positions. By contrast, Cd level in fish liver has varied as C. striata > A. testudineus > E. suratensis . The concentration of Cd in the liver is very low (0.065–0.087 mg/kg) at lower trophic positions and it is relatively higher (0.500–0.536 mg/kg) at higher trophic positions. This indicates an increase in dietary Cd level towards the higher trophic position reflecting the possibility of bioaccumulation of more Cd at higher trophic positions. It also suggests the possibilities of biomagnification of Cd along aquatic food chains. Therefore, Cd pollution is more severe in higher trophic positions.
Previous studies have reported similar observations with respect to As and Cd in fish tissues and stresses that As is bioconcentrated by fish but not biomagnified through the food chain (Eisler, 1988 ; Maeda, 1994 ; Mason et al ., 2000 ; Barwick and Maher, 2003Culioli et al ., 2009 ; Leeves, 2011 ). However, Cd shows biomagnification (Croteau et al ., 2005 ; Abdelghani and Elchaghaby, 2007 ). Therefore, the results of the current study are similar to the above results. Several studies have demonstrated the accumulation tendency of As and Cd in freshwater fish. Reviewing information from thirty-two studies related to Cd accumulation in natural environments, Perera et al. (2015) reported that the liver shows higher affinity (24 studies out of 32) towards Cd rather than kidneys (5 out of 32) and gills (7 out of 32). If As is considered, the accumulation tendency might be highest in the gill (Akan et al ., 2009 ; Petkovšek et al ., 2012 ), kidney (Palaniappan and Vijayasundaram, 2009 ), liver (Culioli et al ., 2009 ; Dsikowitzky et al ., 2013 ) or muscle (Squadrone et al., 2013 ). Therefore, it is apparent that the accumulation pattern of As and Cd in fish tissues can be varied because many other factors in the water can affect bioaccumulation in fish (Iivonen et al ., 1992 ; Mason et al ., 2000 ). However, the presence of As and Cd in river water affects its bioaccumulation in fish.
Relationship between Arsenic and Cadmium in Fish Species and Environmental Compartments
Gill and Kidney
Significant (p < 0.05) positive correlations were observed between water-As and gill-As as well as between water-Cd and gill-Cd levels in all three fish species (Table 7 ). Correlations between sediment-As level and gills of E . suratensis was poor but positive (r = .027, p = 0.899). However, sediment-As level was significantly positively correlated with the gill tissue of A. testudineus (r = .433, p = 0.035) and C. striata (r = .457, p = 0.025). Sediment-Cd level was poorly positively correlated in E. suratensis (r = .014, p = 0.947). The correlation between sediment-Cd level and gill-Cd level in A. testudineus (r = .497, p = 0.007) and C. striata (r = .449, p = 0.028) was significantly positively correlated. The correlation between trace element level between abiotic factors (water and sediment) and kidney tissue showed similar results. Water-As and water-Cd level was poorly positively correlated with As and Cd in the kidneys of all three fish species. Similarly, a poorly positive correlation was observed between sediment-As and sediment-Cd level and kidney of all fish species.
| Species | Tissue | Arsenic | Cadmium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Sediment | Water | Sediment | ||||||||
| n | r | p | r | p | n | r | p | r | p | ||
| E. suratensis | Gill | 24 | .508† | .011 | .027 | .899 | 24 | .475† | .019 | .014 | .947 |
| Kidney | 27 | .068 | .751 | .150 | .486 | 24 | .226 | .289 | .132 | .538 | |
| Liver | 27 | .133 | .536 | .336 | .108 | 24 | .058 | .788 | .331 | .114 | |
| Muscle | 24 | −.272 | .198 | .203 | .340 | 24 | −.189 | .377 | .058 | .789 | |
| A. testudineus | Gill | 24 | .521‡ | .009 | .433† | .035 | 25 | .482† | .017 | .497‡ | .007 |
| Kidney | 27 | .096 | .654 | .118 | .582 | 25 | .256 | .227 | .025 | .909 | |
| Liver | 24 | .193 | .366 | .269 | .204 | 24 | .376 | .070 | .536‡ | .007 | |
| Muscle | 24 | .142 | .507 | .265 | .211 | 24 | .170 | .427 | −.039 | .858 | |
| C. striata | Gill | 24 | .507† | .011 | .457† | .025 | 28 | .652‡ | .001 | .449† | .028 |
| Kidney | 26 | .161 | .452 | .301 | .153 | 26 | .258 | .224 | .451 | .027 | |
| Liver | 24 | .230 | .281 | .276 | .192 | 24 | .288 | .173 | .511† | .011 | |
| Muscle | 24 | .050 | .818 | .400 | .053 | 24 | .351 | .092 | −.038 | .861 | |
p values for each pair of variables are reported; correlation is significant at the 0.05 level (†) and at the 0.01 level (‡).
Arsenic and cadmium was present in variable concentrations at different study sites of the upper Malwathu Oya (Table 3 ; Table 4 ). According to the present data, the level of As and Cd in the gills highly depend on the concentrations of the two elements in the water. Fish do not cohabit at one site but they habitually move from one site to the other. Their gills are continuously exposed to different concentrations of arsenic and cadmium while incessantly moving between sites (i.e., from paddy sites to forest sites and vice versa). Therefore, trace element levels in the gills change according to the available concentrations in the water.
Their gills are continuously exposed to different concentrations of arsenic and cadmium while incessantly moving between sites from paddy sites to forest sites and vice versa . Fish can up-take trace metals by two main routes ( Farkas et al ., 2000 ; Rozon-Ramilo et al ., 2011 ), either from water through the gills or through the digestive tract. However, the significant correlation between sediment trace element levels and gills of A. testudineus and C . striata ( Table 7 ) are related to the feeding habits of these two fish species. A. testudineus ( Riede, 2004 ) and C. striata ( Rahman et al., 2012 ) prefer to access the river bottom. Because of their preference towards animal matter, these two species access the bottom to eat up bottom-living invertebrates (Pethiyagoda, 1991 ). As a result, the bottom sediment, which contains more arsenic and cadmium (Fig. 5 ), becomes agitated. Therefore, when fish are feeding, their gills come into direct contact with arsenic- and cadmium-rich water, which resulted in having more trace elements in the gill tissue. However, a poor correlation between sediment As level and E. suratensis gill As level was observed and it is also associated with the habitat preference of the fish. They prefer to live and feed near the bottom as well as in midwaters or near the surface (benthopelagic). Therefore, the habitat preferences as well as the bioturbation of bottom sediment by fish for diet requirements mix more As and Cd with water, which possibly passes through their gills resulting in more trace elements in the gill tissue. Therefore, the positive relationship between the gill and sediment trace element level is understandable. The behaviour of As and Cd in the kidney seems to be more or less similar. As waterborne exposure results in more As and Cd in the gills and kidneys ( Oladimeji et al ., 1984 ; Szebedinszky et al ., 2001 ; Franklin et al ., 2005 ), their deposition and excretions rates might not be diverse with respect to kidney tissue. Therefore, a similar correlation was observed in all fish species.
Liver
Arsenic and cadmium levels between water and the liver poorly but positively correlated in all three species (Table 7 ). Similarly, sediment-As level was poorly positively correlated with the liver in all species. However, the behaviour of sediment-Cd was rather different; although sediment-Cd level was poorly correlated with the liver-Cd level of E. suratensis (r = 331, p = .114), it showed a significantly positive correlation in A. testudineus (r = 536, p = .007) and C. striata (r = 511, p = .011).
Arsenic and cadmium in water did not significantly affect the level of those in the liver with respect to all three fish species. Similarly, sediment-As content has not significantly affected the liver-As level in E. suratensis , probably due to the fact that E. suratensis mainly prefers plant matter as their food ( Pethiyagoda, 1991 ). Although trace metal accumulation is possible in plant matter, being at the lowest level in aquatic food chains, the available levels might be much lower. In contrast, strong correlation between sediment-Cd level and the liver tissue reflects the feeding habit of A. testudineus and C. striata . They represent higher trophic levels in aquatic food chains and consume worms, crustaceans and molluscs from the river bottom. Therefore, since sediments contains Cd, the potential effect of bioaccumulation is quite possible when consumer organisms subsequently ingest metals bioaccumulated in organisms at a lower trophic levels ( Drexler et al., 2003 ). Therefore, the accumulated rate of Cd in A. testudineus and C. striata might be higher than the depuration rates which resulted in a strong correlation between sediment-Cd level and liver.
Muscle
Arsenic and cadmium levels between water and muscle tissue did not show a significantly positive correlation with all three species (Table 7 ). Similarly, sediment-As level did not positively correlate with the muscle tissue in all species.
Water-As and water-Cd levels did not affect As and Cd levels in the muscle tissue in all fish species. As the trace element level in the muscle tissue is comparatively very low (Table 5 ; Table 6 ), the effect of As and Cd on the muscle tissue can also be low. Poor relationship between sediment-As levels and the muscle tissue indicates that the effect of sediment on muscle-As level in all species is at a minimum level. Similarly, poor correlation between sediment-Cd levels and the muscle tissue of E. suratensis indicates that the effect of sediment on the accumulation of Cd in it is at a minimum level.
Rosso et al. (2013) reported that out of nine fish species, two detritivore fish species (Cyphocharax voga and Astyanax eigenmanniorum ) showed significant correlation coefficients with sediment-As. However, in the same study, they have not observed significant correlation between water and fish. Another study reported that a correlation was not found between Cd concentrations in water and sediment or between Cd concentrations in water and muscle and gills of the fish Leuciscus cephalus ( Demirak et al., 2006 ). Therefore, the negative relationship might be associated with the physiological adaptations of the two fish species. Further, release of trace elements from sediments into the water body and, consequently, to the fish, depends on the chemical fractionation of metals and other factors such as sediment pH and other physical and chemical characteristics of the aquatic system (Morgan and Stumm, 1991 ).
Fish as Water Pollution Indicators
Accumulation of As and Cd in fish tissues can be related to contamination level in water and sediments. In freshwater fish, the gills are the main point of entry for dissolved metals (Li et al., 2009 ) and metalloids (Ananth et al., 2014 ). Therefore, arsenic and cadmium level in gills essentially reflect their potency in the water. It is further emphasized by the significant (p < 0.05) correlation between water and gill arsenic level of all species in the present study as well. Significant (p < 0.01) correlation between water-As and gill-As level of A . testudineus as well as between water-Cd and gill-Cd level of C. striata ( Table 7 ) stresses the affinity of the gill tissue of those two species towards waterborne arsenic and cadmium. If the quantity of a pollutant in the environment or its change can be determined by using a species, it reflects the possibility of that species being used as a biomonitor species (Markert et al., 1997 ). However, considering the present data, except for the gills, all of the tissues studied in the three species do not reflect the trace element profile in water,; thus, only the gills can be considered as a possible bioindicator tissue. Moreover, the gills of A. testudineus and C . striata reflects the pollution level (as correlation was highly significant) of As and Cd, respectively. However, not all species are suitable for use in biomonitoring programs because some species can efficiently regulate their body trace element levels ( Bryan, 1984 ). Exposing the fish to variable levels of the two trace elements, the possibility of their reflecting the quality of the environment should be monitored. Therefore, further studies are required to understand the suitability of these two species in biomonitoring programs.
Suitability of Fish for Human Consumption
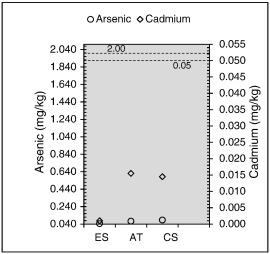
E. suratensis , A. testudineus and C. striata are more common local food fishes. Overall As and Cd levels in these fish species are shown in Fig. 6 . In all four tissues, As concentration ranged from 0.026 to 3.438 mg/kg, 0.064 to 4.732 mg/kg and 0.072 to 4.321 mg/kg in E. suratensis , A. testudineus and C. striata , respectively ( Table 5 ). Cadmium varies from 0.001 to 0.110 mg/kg, 0.004 to 0.395 mg/kg and 0.005 to 0.536 mg/kg in E. suratensis , A . testudineus and C. striata , respectively ( Table 6 ). Nevertheless, being the consumable portion of the fish, identification of trace metal levels in muscle meat is essentially useful.
|
|
|
Fig. 6. Arsenic and cadmium level in the muscle tissue (horizontal dotted lines indicate maximum permissible levels). |
According to the above results, As and Cd concentrations in the muscle meat of all three fish species were well below the maximum permissible levels of many recognized international institutions (Fig. 6 ). For example, according to Australia New Zealand Food Authority (1999) the recommended arsenic level is 2.0 μg/g and it is 1.2 μg/g for the USEPA (2000) . According to the European Union (2006) and (MOFAR (1996) of Sri Lanka, the maximum suggested level of cadmium for human consumption is 0.05 mg/kg in wet weight basis of the fish muscles.
Arsenic occurs in different inorganic and organic forms in the environment, both from natural occurrence and from anthropogenic sources. The inorganic forms of arsenic are more toxic as compared to the organic arsenic (EFSA, 2009 ) and are mostly found in surface waters as oxyanions of trivalent arsenite (As(III)) or pentavalent arsenate (As(V)) (Smedley and Kinniburgh, 2002 ). Organic forms of arsenic (e.g., monomethylarsonic acid and dimethylarsinic acid) are usually minor in surface waters but proportions of organic forms of arsenic can increase as a result of methylation reactions catalysed by microbial activity (IPCS, 2001 ; Smedley and Kinniburgh, 2002 ). In the environment, As behaves in similar ways to nitrogen and phosphorus (Smedley and Kinniburgh, 2002 ). As a result of these similarities, arsenic gets taken into the biochemical pathways of nitrogen and phosphorus, resulting in the formation of compounds such as arsenobetaine in fish (Wahlen, 2004 ). Higher percentage of As in fish is present as organic As (Mandal and Suzuki, 2002 ; Ciardullo et al ., 2010 ). These organic forms become toxic after metabolic conversion to the trivalent form of arsenic (IPCS, 2001 ). However, inorganic arsenic compounds are also found in fish (Ciardullo et al., 2010 ).
The FAO/WHO Expert Committee on Food Additives (JECFA, 2010 ) derived an inorganic arsenic BMDL (benchmark dose) for a 0.5% increased incidence of lung cancer, which was computed to be 3.0 μg/kg body weight per day. Average human body mass globally was 62.0 kg and 57.7 kg in Asia (Walpole et al., 2012 ), thus the average body mass of a Sri Lankan is considered as 60 kg. Therefore, 180 μg of arsenic per day is allowable in an adult person. As the per capita fish consumption in Sri Lanka is 36 g per day (Amarasinghe, 2013 ), by consumption of E. suratensis (mean arsenic level is 0.045 mg/kg), a person can receive a maximum of 1.62 μg of arsenic per day (Table 8 ). Therefore, the level derived from the current study with respect to E. suratensis (1.62 mg/day) is well below the recommended levels of (JECFA, 2010 ). Similarly, JECFA (2010) derived BMDL for cadmium to be 0.80 μg/kg body weight per day (or 25 μg/kg body weight per month (PTMI).2 By consuming E. suratensis (mean cadmium level is 0.001 mg/kg), a person receives 0.036 μg of cadmium per day. The estimation of arsenic and cadmium for other species is given in Table 8 .
| Fish species | Arsenic (μg/day) | Cadmium (μg/day) |
|---|---|---|
| E. suratensis | 1.620 | 0.036 |
| A. testudineus | 2.628 | 0.540 |
| C. striata | 3.096 | 0.504 |
A limited number of other studies have demonstrated As and Cd in the muscle meat of different freshwater fish species in Sri Lanka. Jinadasa et al. (2013) reported very low concentrations (< 0.05 mg/kg) of Cd in the muscle meat of common freshwater food fishes (Oreochromis spp. and C. striata ) collected from the NCP. However, a considerable level of As was not detected in the above study. Indrajith et al. (2010) reported 0.002–0.048 mg/kg in the muscle tissue of E. suratensis . The first study was conducted with specimens collected during eight months from six reservoirs of the Anuradhapura District (in NCP) while the latter was restricted to specimens collected from several locations within several months (exact duration not given). Out of the limited number of studies conducted with respect to the bioavailability of trace elements in freshwater fishes in the NCP, being the most marketable and abundant species, a majority of studies address arsenic and cadmium in exotic species (Oreochromis mossambicus and Oreochromis niloticus ). For example, Rajapaksha et al. (2008) reported 0.06–0.20 mg/kg cadmium in the muscle tissue of O. niloticus collected from two reservoirs in Anuradhapura District in 2004 (exact duration not given). Chronic kidney disease with uncertain aetiology being one of the most controversial current health topics in the country, and some schools of thought indicate possible correlation with trace elements and CKDu, assessment of tissue level As bioaccumulation should be closely monitored. The reported negative results of arsenic might be associated with the restriction of sampling for several months without continuous monitoring of specimens. In contrast, the current study continued for 43 months (in a single reservoir) by collecting fish samples (from three fish species) on a monthly basis (fish). Therefore, the observed results of the current study might be different from others. However, the observed trace element levels of the current study are within the suggested safe range. It emphasizes that the muscle meat of the three fish species found in upper Malwathu Oya is safe for human consumption.
Conclusions
Paddy cultivation has been identified as the main anthropogenic source that affects river water quality. Arsenic and cadmium concentration in water showed a bimodal pattern which coincided with the bimodal rainfall of the dry zone and the paddy cultivation pattern in the area. The presence of arsenic and cadmium in sediments and water affect bioaccumulation in fish. However, the trace element levels in fish varied as C. striata > A. testudineus > E. suratensis. Accumulation of As does not change according to the feeding habits of fish. Nevertheless, the feeding habits and habitat preferences of fish affect the accumulation tendency of Cd. Arsenic accumulation pattern in different organs (i.e., kidney > liver > gill > muscle) stresses that the waterborne pathway is more important and Cd accumulation pattern (i.e., liver > kidney > gill > muscle) reflects the importance of dietary pathways. Further comprehensive assessment on the gills of A. testudineus and C. striata is necessary to understand the suitability of the species for biomonitoring studies. Water and sediment trace element levels do not considerably affect the muscle tissue. Therefore, fish consumption is not affected by paddy cultivation.
References
- Abdelghani and Elchaghaby, 2007 N.T. Abdelghani, G.A. Elchaghaby; Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ions from waste water by adsorption; Int. J. Environ. Sci. Technol., 4 (2007), pp. 451–456
- Akan et al., 2009 J.C. Akan, F.I. Abdulrahman, O.A. Sodipo, P.I. Akandu; Bioaccumulation of Some Heavy Metals of Six Fresh Water Fishes Caught from Lake Chad in Doron Buhari, Maiduguri, Borno State, Nigeria; 4 (2009), pp. 103–114
- Alhashemi et al., 2012 H. Alhashemi, M. Sekhavatjou, B.H. Kiabi, A.R. Karbassi; Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland, Iran; Microchem. J., 104 (2012), pp. 1–6 http://dx.doi.org/10.1016/j.microc.2012.03.002
- Ali, 1990 A.B. Ali; Rice/fish farming in Malaysia: a resource optimization; Ambio, 19 (1990), pp. 404–408
- Amarasinghe, 2013 U.S. Amarasinghe; Fisheries resources in alleviation of hunger and malnutrition in Sri Lanka—accomplishment and challenges; Sri Lanka J. Aquat. Sci., 18 (2013), pp. 1–15
- Amarasinghe and Silva, 1999 U.S. Amarasinghe, S.S.D. Silva; The Sri Lankan reservoir fishery: a case for introduction of a co-management strategy; Fish. Manag. Ecol., 6 (1999), pp. 387–399
- Ambedkar and Muniyan, 2011 G. Ambedkar, M. Muniyan; Bioaccumulation of some Heavy Metals in the selected five freshwater fish from Kollidam River; 2, Tamilnadu, India (2011), pp. 221–225
- Amiard et al., 1987 J.C. Amiard, C. Amiard-Triquet, B. Berthet, C. Métayer; Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms; J. Exp. Mar. Biol. Ecol., 106 (1987), pp. 73–89
- Ananth et al., 2014 S. Ananth, V. Mathivanan, S. Aravinth, V. Sangeetha; Impact of arsenic metal toxicant on biochemical changes in the grass carp, Ctenopharyngodon idella; Int. J. Mod. Res. Rev., 2 (2014), pp. 74–78
- APHA, 2005 APHA; Standard methods for the examination of water and wastewater; (21st ed)American Public Health Association, Washington DC. (2005)
- Australia New Zealand Food Authority, 1999 Australia New Zealand Food Authority; Food Standards Code in the Gazette; Australia New Zealand Food Authority (1999)
- Barwick and Maher, 2003 M. Barwick, W. Maher; Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia; Mar. Environ. Res., 56 (2003), pp. 471–502 http://dx.doi.org/10.1016/S0141-1136(03)00028-X
- Bryan, 1984 G.W. Bryan; Pollution due to heavy metals and their compounds; Mar. Ecol., 5 (1984), pp. 1289–1431
- Canli and Atli, 2003 M. Canli, G. Atli; The relationships between heavy metals (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species; Environ. Pollut., 121 (2003), pp. 129–136
- Canli et al., 1998 M. Canli, Ö Ay, M. Kalay; Levels of Heavy Metals (Cd, Pb, Cu, Cr and Ni) in Tissue of Cyprinus carpio; 22, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey (1998), pp. 149–157
- Chen et al., 2015 H. Chen, Y. Teng, S. Lu, Y. Wang, J. Wang; Contamination features and health risk of soil heavy metals in China; Sci. Total Environ., 512–513 (2015), pp. 143–153 http://dx.doi.org/10.1016/j.scitotenv.2015.01.025 (ISSN 0048-9697)
- Cheung et al., 2003 K.C. Cheung, B.H.T. Poon, C.Y. Lan, M.H. Wong; Assessment of metal and nutrient concentrations in river water and sediment collected from the cities in the Pearl River Delta, South China; Chemosphere, 52 (2003), pp. 1431–1440 http://dx.doi.org/10.1016/S0045-6535(03)00479-X
- Ciardullo et al., 2010 S. Ciardullo, F. Aureli, A. Raggi, F. Cubadda; Arsenic speciation in freshwater fish: focus on extraction and mass balance; Talanta, 81 (2010), pp. 213–221
- Costa, 1983 H.H. Costa; Biological studies of the pearl spot E troplus suratensis (Pisces, Cichlidae) from three different habitats in Sri Lanka ; Int. Rev. der gesamten Hydrobiol. und Hydrogr., 68 (1983), pp. 565–580 http://dx.doi.org/10.1002/iroh.19830680408
- Courtenay and Williams, 2004 W.R. Courtenay, J.D. Williams; Snakeheads (Pisces, Channidae): A Biological Synopsis and Risk Assessment; US Geological Survey (2004)
- Croteau et al., 2005 M. Croteau, S.N.S. Luoma, A.R. Stewart; Trophic transfer of metals along freshwater food webs: evidence of cadmium biomagnification in nature; Limnol. Oceanogr., 50 (2005), pp. 1511–1519 http://dx.doi.org/10.4319/lo.2005.50.5.1511
- Culioli et al., 2009 J.-L. Culioli, S. Calendini, C. Mori, A. Orsini; Arsenic accumulation in a freshwater fish living in a contaminated river of Corsica, France; Ecotoxicol. Environ. Saf., 72 (2009), pp. 1440–1445 http://dx.doi.org/10.1016/j.ecoenv.2009.03.003
- Delmotte et al., 2007 S. Delmotte, F.J.R. Meysman, A. Ciutat, A. Boudou, S. Sauvage, M. Gerino; Publication: cadmium transport in sediments by tubificid bioturbation: an assessment of model complexity; Geochim. Cosmochim. Acta, 71 (2007), pp. 844–862 http://dx.doi.org/10.1016/j.gca.2006.11.007
- Demirak et al., 2006 A. Demirak, F. Yilmaz, A.L. Tuna, N. Ozdemir; Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey ; Chemosphere, 63 (2006), pp. 1451–1458 http://dx.doi.org/10.1016/j.chemosphere.2005.09.033
- Department of Agriculture, 2006 Department of Agriculture; Climate of Rice Growing Regions in Sri Lanka [WWW Document]; URL http://www.doa.gov.lk/index.php/en/crop-recommendations/903 (2006) (accessed 1.10.16)
- Department of Meteorology, 2014 Department of Meteorology; Climate in Sri Lanka [WWW Document]; URL http://www.meteo.gov.lk/index.php?option=com_content&view=article&id=13&Itemid=132&lang=en (2014) (accessed 10.15.15)
- Dissanayake and Chandrajith, 2009 C.B. Dissanayake, R. Chandrajith; Phosphate mineral fertilizers, trace metals and human health; J. Natl. Sci. Found. Sri Lanka, 37 (2009), pp. 153–165
- Drexler et al., 2003 J. Drexler, N. Fisher, G. Henningsen, R. Lanno, J. Mcgeer, K. Sappington; Issue Paper on the Bioavailability and Bioaccumulation of Metals. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC., USA; (2003)
- Dsikowitzky et al., 2013 L. Dsikowitzky, M. Mengesha, E. Dadebo, C.E.V. de Carvalho, S. Sindern; Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley lakes, Ethiopia; Environ. Monit. Assess., 185 (2013), pp. 3117–3131 http://dx.doi.org/10.1007/s10661-012-2777-8
- Edem et al., 2008 C.A. Edem, S.B. Akpan, M.I. Dosunmu; A comparative assessment of heavy metals and hydrocarbon accumulation in Sphyrena afra , Orechromis niloticus and Elops lacerta from Anantigha Beach market in Calabar-Nigeria ; Afr. J. Environ. Pollut. Health, 6 (2008), pp. 61–64
- EFSA, 2009 EFSA; Scientific opinion on arsenic in food 1; EFSA J., 7 (2009), p. 1351 http://dx.doi.org/10.2903/j.efsa.2009.1351
- Eisler, 1988 R. Eisler; Cadmium hazards to reviews fish, wildlife, and invertebrates: a synoptic review. US Fish and Wildlife Service Contaminant Hazard Reviews; 85 (12) (1988), pp. 1–65 http://catalogue.nla.gov.au/Record/4005011
- El-Moselhy et al., 2014 K.M. El-Moselhy, A.I. Othman, H. Abd El-Azem, M.E.A. El-Metwally; Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt; Egypt. J. Basic Appl. Sci., 1 (2014), pp. 97–105 http://dx.doi.org/10.1016/j.ejbas.2014.06.001
- Eneji et al., 2011 I.S. Eneji, R. Sha'Ato, P.A. Annune; Bioaccumulation of heavy metals in fish (Tilapia zilli and Clarias gariepinus ) organs from river Benue, North-Central Nigeria ; Pak. J. Anal. Environ. Chem., 12 (2011), pp. 25–31
- EPA, 1990 EPA; Specifications and Guidance for Obtaining Contaminant-Free Sample Containers; U.S. Environmental Protection Agency (1990) OSWER Directive #93240.0-05
- EPA, 1997 EPA; Volunteer Stream Monitoring: A Methods Manual; (1997)
- European Union, 2006 European Union; Commission Regulation No. 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs; (2006)
- Extension Toxicology Network, E.T, 1993 Extension Toxicology Network, E.T; Bioaccumulation; http://extoxnet.orst.edu/tibs/bioaccum.htm (1993) (accessed 2015/11/04)
- Farkas et al., 2000 A. Farkas, J. Salánki, I. Varanka; Heavy metal concentrations in fish of Lake Balaton; Lakes Reserv. Res. Manag., 5 (2000), pp. 271–279 http://dx.doi.org/10.1046/j.1440-1770.2000.00127.x
- Franklin et al., 2005 N.M. Franklin, C.N. Glover, J.A. Nicol, C.M. Wood; Calcium/cadmium interactions at uptake surfaces in rainbow trout: waterborne versus dietary routes of exposure; Environ. Toxicol. Chem., 24 (2005), pp. 2954–2964
- Godt et al., 2006 J. Godt, F. Scheidig, C. Grosse-Siestrup, V. Esche, P. Brandenburg, A. Reich, D.A. Groneberg; The toxicity of cadmium and resulting hazards for human health; J. Occup. Med. Toxicol., 1 (2006), p. 22 http://dx.doi.org/10.1186/1745-6673-1-22
- Govind and Madhuri, 2014 P. Govind, S. Madhuri; Heavy metals causing toxicity in animals and fishes; Res. J. Anim. Vet. Fish. Sci., 2 (2014), pp. 17–23
- Hooda, 2010 P.S. Hooda; Front Matter; P. Hooda (Ed.), Trace Elements in Soils, John Wiley and Sons, Ltd., New York, USA, USA (2010)
- Houserová et al., 2007 P. Houserová, V. Kubán, S. Krácmar, J. Sitko; Total mercury and mercury species in birds and fish in an aquatic ecosystem in the Czech Republic; Environ. Pollut., 145 (2007), pp. 185–194 http://dx.doi.org/10.1016/j.envpol.2006.03.027
- Iivonen et al., 1992 P. Iivonen, S. Piepponen, M. Verta; Factors affecting trace-metal bioaccumulation in Finnish headwater lakes; Environ. Pollut., 78 (1992), pp. 87–95
- Illeperuma, 2000 O.A. Illeperuma; Environmental pollution in Sri Lanka: a review; J. Natl. Sci. Found. Sri Lanka, 28 (2000), pp. 301–325
- Indrajith et al., 2010 H. Indrajith, K. Pathiratne, A. Pathiratne; Heavy metal levels in two food fish species from Negombo estuary, Sri Lanka: relationships with the body size; Sri Lanka J. Aquat. Sci., 13 (2010), pp. 63–81 http://dx.doi.org/10.4038/sljas.v13i0.2207
- IPCS, 2001 IPCS; Arsenic and arsenic compounds; Geneva, World Health Organization, International Programme on Chemical Safety (2nd ed) (2001) (Environmental Health Criteria 224; http://whqlibdoc.who.int/ehc/WHO_EHC_224.pdf )
- Jauasumana et al., 2011 M.A.C.S. Jauasumana, P.A. Paranagama, M. Amarasinge, S.J. Fonseka, D.V. Wijekoon; Presence of Arsenic in Pesticides Used in Sri Lanka; Water Professionals Day Symposium, Water Resources Research in Sri Lanka, Faculty of Agriculture, University of Peradenyia (2011), pp. 127–141
- Jayasumana et al., 2014a C. Jayasumana, R. Gajanayake, S. Siribaddana; Importance of Arsenic and Pesticides in Epidemic Chronic Kidney Disease in Sri Lanka; 15 (2014), pp. 1–5 http://dx.doi.org/10.1186/1471-2369-15-124
- Jayasumana et al., 2014b C. Jayasumana, S. Gunatilake, P. Senanayake; Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka?; 11 (2014), pp. 2125–2147 http://dx.doi.org/10.3390/ijerph110202125
- JECFA, 2010 JECFA; Summary of Conclusion of the Sixty Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives; (2010) (Rome, Italy, Italy)
- Jezierska and Witeska, 2006a B. Jezierska, M. Witeska; Soil and Water Pollution Monitoring, Protection and Remediation, NATO Science Series; Springer Netherlands, Dordrecht (2006) http://dx.doi.org/10.1007/978-1-4020-4728-2
- Jezierska and Witeska, 2006b B. Jezierska, M. Witeska; The metal uptake and accumulation in fish living in polluted waters; Soil Water Pollut. Monit. Prot. Remediat. (2006), pp. 3–23 http://dx.doi.org/10.1007/978-1-4020-4728-2_6
- Jinadasa et al., 2013 B.K.K.K. Jinadasa, M.M. Subasinghe, K. Thayalan, I. Wickramasinghe, M.S.W.D. Silva; Trace Metal Contents in Muscle Tissues of Inland Fish Species in the North Central Province of Sri Lanka; 42 (2013), pp. 79–86
- Joseph and Joseph, 1988 P.S. Joseph, M.M. Joseph; Feeding Habits of the Pearl Spot Etroplus suratensis (Bloch) in the Nethravati–Gurpur Estuary ; The First Indian Fisheries Forum Proceedings (1988), pp. 203–206
- Kabata et al., 2016 R. Kabata, S. Nanayakkara, S. Senevirathna, K.H. Harada, R. Chandrajith, T. Hitomi, T. Abeysekera, T. Takasuga, A. Koizumi; Neonicotinoid concentrations in urine from chronic kidney disease patients in the north central region of Sri Lanka; J. Occup. Health, 58 (2016), pp. 128–133 http://dx.doi.org/10.1539/joh.15-0140-BR
- Kamunde and MacPhail, 2011 C. Kamunde, R. MacPhail; Metal–metal interactions of dietary cadmium, copper and zinc in rainbow trout, Oncorhynchus mykiss; Ecotoxicol. Environ. Saf., 74 (2011), pp. 658–667 http://dx.doi.org/10.1016/j.ecoenv.2010.10.016
- Kotze, 1997 P.J. Kotze; Aspects of Water Quality, Metal Contamination of Sediment and Fish in the Olifants River, Mpumalanga; Rand Afr. Univ., South Africa (1997)
- Kumar and Singh, 2010 P. Kumar, A. Singh; Cadmium toxicity in fish: an overview; GERF Bull. Biosci., 1 (2010), pp. 41–47
- Leeves, 2011 S. Leeves; Bioaccumulation of Arsenic, Cadmium, Mercury, Lead and Selenium in the Benthic and Pelagic Food Chain of Lake Baikal; Norwegian University of Science and Technology (2011)
- Li et al., 2009 H. Li, K. Mai, Q. Ai, C. Zhang, L. Zhang; Effects of dietary squid viscera meal on growth and cadmium accumulation in tissues of large yellow croaker, Pseudosciaena crocea R ; Front. Agric. China, 3 (2009), pp. 78–83
- Liao et al., 2003 C.M. Liao, B.C. Chen, S. Singh, M.C. Lin, C.W. Liu, B.C. Han; Acute toxicity and bioaccumulation of arsenic in tilapia (Oreochromis mossambicus ) from a Blackfoot disease area in Taiwan ; Environ. Toxicol., 18 (2003), pp. 252–259 http://dx.doi.org/10.1002/tox.10122
- Luoma and Rainbow, 2005 S.N. Luoma, P.S. Rainbow; Why is metal bioaccumulation so variable? Biodynamics as a unifying concept; Environ. Sci. Technol., 39 (2005), pp. 1921–1931 http://dx.doi.org/10.1021/es048947e
- Luoma and Rainbow, 2008 S.N. Luoma, P.S. Rainbow; Metal Contamination in Aquatic Environments: Science and Lateral Management; (2011th ed.)Cambridge University Press, Cambridge (2008)
- Maeda, 1994 S. Maeda; Arsenic in the Environment, Part 1, Cycling and Characterization; J.O. Nriagu (Ed.), Arsenic in the Environment, John Wiley & Sons Inc. (1994), pp. 155–187
- Mandal and Suzuki, 2002 B.K. Mandal, K.T. Suzuki; Arsenic round the world: a review; Talanta, 58 (2002), pp. 201–235
- Marcovecchio, 2004 J.E. Marcovecchio; The use of Micropogonias furnieri and Mugil liza as bioindicators of heavy metals pollution in La Plata river estuary, Argentina ; Sci. Total Environ., 323 (2004), pp. 219–226
- Markert et al., 1997 B. Markert, J. Oehlmann, M. Roth; General Aspects of Heavy Metal Monitoring by Plants and Animals; K.S. Iyengar Subramanian, G.V. (Eds.), Environmental Biomonitoring: Exposure Assessment and Specimen Banking. Washington (1997), pp. 18–29
- Mason et al., 2000 R.P. Mason, J. Laporte, S. Andres; Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish; Arch. Environ. Contam. Toxicol., 38 (2000), pp. 283–297
- Mat Jais, 2007 A.M. Mat Jais; Pharmacognosy and pharmacology of Haruan (Channa striatus ), a medical fish with wound healing properties ; Bol. Latinoam. y del Caribe Plantas Med. y Aromat., 6 (2007), pp. 52–60 http://dx.doi.org/10.3923/ajava.2013.369.375
- McIntyre and Linton, 2011 D.O. McIntyre, T.K. Linton; Homeostasis and Toxicology of Non-Essential Metals, Fish Physiology; Elsevier (2011) http://dx.doi.org/10.1016/S1546-5098(11)31028-X
- Mermut et al., 1996 A.R. Mermut, J.C. Jain, L. Song, R. Kerrich, L. Kozak, S. Jana; Trace element concentrations of selected soils and fertilizers in Saskatchewan, Canada; J. Environ. Qual., 25 (1996), p. 845 http://dx.doi.org/10.2134/jeq1996.00472425002500040028x
- Meybeck et al., 1992 M. Meybeck, G. Friedrich, R. Thomas, D. Chapman; Water Quality Assessments—A Guide to Use of Biota, Sediments and Water in Environmental Monitoring; D. Chapman (Ed.), Rivers (1992)
- MOFAR, 1996 MOFAR; Fisheries and Aquatic Resource Act, No. 2 of 1996. Colombo, Sri Lanka: The Gazette of the Democratic Socialist Republic of Sri Lanka (No. 1528/7); (1996)
- Mohamed, 2008 F.A.S. Mohamed; Bioaccumulation of selected metals and histopathological alterations in tissues of Oreochromis niloticus and Lates niloticus from Lake Nasser, Egypt ; Glob. Vet., 2 (2008), pp. 205–218
- Morgan and Stumm, 1991 J.J. Morgan, W. Stumm; Chemical Processes in the Environment, Relevance of Chemical Speciation; E. Merien (Ed.), Metals and Their Compounds in the Environment, VCH Publishers, Berlin (1991), pp. 67–103
- Oladimeji et al., 1984 A.A. Oladimeji, S. Qadri, A.S. Defreitas; Measuring the elimination of arsenic by the gills of the rainbow trout (Salmo gairdieri ) by using a two compartment respirator ; Bull. Environ. Contam. Toxicol., 32 (1984), pp. 661–669
- Padmajani et al., 2014 M.T. Padmajani, M.M.M. Aheeyar, M.M.M. Bandara; Assessment of Pesticide Usage in Up-Country Vegetable Farming in Sri Lanka; HARTI Research Report no: 164, Hector Kobbekaduwa Agrarian Research and Training Institute, Colombo, Sri Lanka (2014)
- Palaniappan and Vijayasundaram, 2009 P.L.R.M. Palaniappan, V. Vijayasundaram; The Bioaccumulation of Arsenic and the Efficacy of Meso − 2, 3-Dimercaptosuccinic Acid in the Selected Organ Tissues of Labeo rohita Fingerlings Using Inductively Coupled Plasma–Optical Emission Spectrometry ; 6 (2009), pp. 1247–1254
- Paranagama, 2013 P.A. Paranagama; Potential Link between Ground Water Hardness, Arsenic Content and Prevalence of CKDu; Proceedings of the Symposium on Chronic Kidney Disease of Uncertain Aetiology (CKDu): A Scientific Basis for Future Action, National Academy of Sciences of Sri Lanka (2013), p. 19
- Patrick, 2003 L. Patrick; Toxic metals and antioxidants: part II. The role of antioxidants in arsenic and cadmium toxicity; Altern. Med. Rev., 8 (2003), pp. 106–128
- Perera et al., 2014 P.A.C.T. Perera, T.V. Sundarabarathy, T. Sivananthawerl, U. Edirisinghe; Seasonal variation of water quality parameters in different geomorphic channels of the upper Malwathu Oya in Anuradhapura, Sri Lanka; Trop. Agric. Res., 25 (2014), pp. 158–170
- Perera et al., 2015 P.A.C.T. Perera, P.S. Kodithu, T.V.V. Sundarabarathy, U. Edirisingh, S.P. Kodithuwakku, T.V.V. Sundarabarathy, U. Edirisinghe; Bioaccumulation of cadmium in freshwater fish: an environmental perspective; Insight Ecol., 4 (2015), pp. 1–12 http://dx.doi.org/10.5567/ECOLOGY-IK.2015.1.12
- Pethiyagoda, 1991 R. Pethiyagoda; Freshwater Fishes of Sri Lanka; The Wildlife Heritage Trust of Sri Lanka, Colombo (1991)
- Petkovšek et al., 2012 A.S. Petkovšek, S.M. Grudnik, Z.P. Boštjan; Heavy metals and arsenic concentrations in ten fish species from the Šalek Lakes (Slovenia): assessment of potential human health risk due to fish consumption; Environ. Monit. Assess., 184 (2012), pp. 2647–2662 http://dx.doi.org/10.1007/s10661-011-2141-4
- Punyawardena, 2004 B.V.R. Punyawardena; Technical Report on the Characterization of the Agro-Ecological Context in which Farm Animal Genetic Resources (FAnGR) Are Found: Sri Lanka; (2004)
- Rahman et al., 2012 M.A. Rahman, A. Arshad, S.M.N. Amin; Growth and production performance of threatened snakehead fish, Channa striatus (Bloch), at different stocking densities in earthen ponds ; Aquac. Res., 43 (2012), pp. 297–302
- Rajapaksha et al., 2008 T. Rajapaksha, K.H. Bandara, J.M.R.S. Senevirathna, D.M.A.N. Dasanayake, D.M.R.S.B. Herath, V. Bandara, J.M.R.P. Abeysekara; Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (tilapia); Environ. Geochem. Health, 30 (2008), pp. 465–478
- Riede, 2004 K. Riede; Global report of migratory species – from global to regional scales; Final report of the R&D –Project 808 05 081, Federal Agency for Nature Conservation, Bonn, Germany (2004), p. 329
- Rosso et al., 2013 J.J. Rosso, N.F. Schenone, A.P. Carrera, A.F. Cirelli; Concentration of arsenic in water, sediments and fish species from naturally contaminated rivers; Environ. Geochem. Health, 35 (2013), pp. 201–214 http://dx.doi.org/10.1007/s10653-012-9476-9
- Rozon-Ramilo et al., 2011 L.D. Rozon-Ramilo, M.G. Dubé, A.J. Squires, S. Niyogi; Examining waterborne and dietborne routes of exposure and their contribution to biological response patterns in fathead minnow (Pimephales promelas ) ; Aquat. Toxicol., 105 (2011), pp. 466–481 http://dx.doi.org/10.1016/j.aquatox.2011.07.006
- Sandnes et al., 2000 J. Sandnes, F. Thomas, R. Hansen, B. Sandnes; Influence of particle type and faunal activity on mixing of di(2-ethylhexy1)phthalate (DEHP) in natural sediments; Mar. Ecol. Prog. Ser., 197 (2000), pp. 151–167
- Selvarajah and Thiruchelvam, 2007 A. Selvarajah, S. Thiruchelvam; Factors affecting pesticide use by farmers in Vavuniya District; Trop. Agric. Res., 19 (2007), pp. 380–388
- Senthil et al., 2004 M.S. Senthil, R. Karuppasamy, K. Poongodi, S. Puranesurin; Bioaccumulation pattern of zinc in freshwater fish Channa punctatus (Bloch) after chronic exposure ; Turk. J. Fish. Aquat. Sci., 8 (2004), pp. 55–59
- Smedley and Kinniburgh, 2002 P.L. Smedley, D.G. Kinniburgh; A review of the source, behaviour and distribution of arsenic in natural waters; Appl. Geochem., 17 (5) (2002), pp. 517–568
- Squadrone et al., 2013 S. Squadrone, M. Prearo, P. Brizio, S. Gavinelli, M. Pellegrino, T. Scanzio, S. Guarise, A. Benedetto, M.C. Abete; Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis ) from Italian rivers ; Chemosphere, 90 (2013), pp. 358–365 http://dx.doi.org/10.1016/j.chemosphere.2012.07.028
- Stednick, 1991 J.D. Stednick; Wildland Water Quality Sampling and Analysis; Harcourt Brace Jovanovich Publishers, San Diego, California (1991)
- Subathra and Karuppasamy, 2007 S. Subathra, R. Karuppasamy; Toxic effects of copper on bioenergetics and growth rates in fingerlings and adult age of the fish, Mystus vittatus (Bloch, 1794) ; J. Fish. Aquat. Sci., 2 (2007), pp. 285–293 http://dx.doi.org/10.3923/jfas.2007.285.293
- Szebedinszky et al., 2001 C. Szebedinszky, J.C. McGeer, D.G. McDonald, C.M. Wood; Effects of chronic Cd exposure via the diet or water on internal organ-specific distribution and subsequent gill Cd uptake kinetics in juvenile rainbow trout (Oncorhynchus mykiss ) ; Environ. Toxicol. Chem., 20 (2001), pp. 597–607
- Taweel et al., 2011 A. Taweel, M. Shuhaimi-Othman, A.K. Ahmad; Heavy Metals Concentration in Different Organs of Tilapia Fish (Oreochromis niloticus ) from Selected Areas ; 10 (2011), pp. 11562–11566 http://dx.doi.org/10.5897/AJB11.1663
- The National Atlas of Sri Lanka, 2007 The National Atlas of Sri Lanka; (second ed.)Survey Department of Sri Lanka (2007)
- USEPA, 2000 USEPA; Guidance for Assessing Chemical Contaminant, Data for Use in Fish Advisories, Volume 2. Risk Assessment and Fish Consumption Limits; (third ed) (2000) (DC: Washington)
- Vidhya and Nair, 2012 V. Vidhya, C.R. Nair; Etroplus suratensis (Bloch) in Rajakkamangalam estuary ; Int. J. Cur. Tr. Res, 1 (2012), pp. 107–109
- Vidhya and Radhakrishnan, 2012 V. Vidhya, N. Radhakrishnan; Observations of food and feeding habits of Etroplus suratensis (Bloch) in Rajakkamangalam estuary ; Int. J. Cur. Tr. Res, 1 (2012), pp. 107–109
- Wahlen, 2004 R. Wahlen; Fast and accurate determination of arsenobetaine in fish tissues using accelerated solvent extraction and HPLC-ICP-MS determination; J. Chromatogr. Sci., 42 (4) (2004), pp. 217–222 (PubMed PMID: 15154985)
- Walpole et al., 2012 S.C. Walpole, D. Prieto-Merino, P. Edwards, J. Cleland, G. Stevens, I. Roberts; The weight of nations: an estimation of adult human biomass; BMC Public Health, 12 (2012), pp. 1–6 http://dx.doi.org/10.1186/1471-2458-12-439
- Wang et al., 2012 S. Wang, L. Xu, Z. Zhao, S. Wang, Y. Jia, H. Wang, X. Wang; Arsenic retention and remobilization in muddy sediments with high iron and sulfur contents from a heavily contaminated estuary in China; Chem. Geol., 314-317 (2012), pp. 57–65 http://dx.doi.org/10.1016/j.chemgeo.2012.05.005
- WHO, 2006 WHO; Guidelines for Drinking-Water Quality—Vol. 1; (2006)
- Wijesundara et al., 2013 W.M.G.D. Wijesundara, W.M.G.D. Nandasena, A.N. Jayakody; Seasonal and spatial variations of N, P, K and Cd concentrations in water of the Mahakanumulla cascade in the dry zone of Sri Lanka; Trop. Agric. Res., 24 (2013), pp. 279–288
- Wimalawansa, 2015a S.J. Wimalawansa; The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication; Environ. Geochem. Health (2015) http://dx.doi.org/10.1007/s10653-015-9768-y
- Wimalawansa, 2015b S.J. Wimalawansa; Agrochemicals and chronic kidney disease of multi-factorial origin (CKDmfo): an environmentally induced, occupational exposure disease; Int. J. Nephrol Kidney Fail, 1 (2015) http://dx.doi.org/10.16966/2380-5498.111
- Zalina et al., 2012 I. Zalina, C.R. Saad, A. Christianus, S.A. Harmin; Induced breeding and embryonic development of climbing perch (Anabas testudineus , Bloch) ; J. Fish. Aquat. Sci., 7 (2012), pp. 291–306 http://dx.doi.org/10.5567/ECOLOGY-IK.2013.8.14
- Zielinski et al., 2003 P. Zielinski, A. Gorniak, T. Suchowolec; P. Zielinski, A. Gorn, T. Suchowolec (Eds.), Changes in Water Chemistry Along the Course of Two Rivers with Different Hydrological Regimes (2003), pp. 111–117
Notes
1. CKDu, chronic kidney disease of unknown aetiology.
2. Provisional Tolerable Monthly Intake
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?