m (Tag: Visual edit) |
|||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 20: | Line 20: | ||
*Corresponding address: ''lihaigang2024[mailto:@ @]163.com (Haigang Li)''</div> | *Corresponding address: ''lihaigang2024[mailto:@ @]163.com (Haigang Li)''</div> | ||
--> | --> | ||
| + | ==Abstract== | ||
| − | + | This paper proposes a self-filling exhaust-type natural vacuum solar distillation system for seawater desalination, which aligns with China's "green, low-carbon, and efficient" strategy and its "carbon peak and carbon neutrality" goal. The system’s structure and working principle are described, and an experimental apparatus is built to test its water production at different temperatures. The results show that the water production and performance coefficient increase with the water temperature. At a seawater heating mass flow rate of 0.1 kg/s and a temperature of 60℃, the unit cycle water production is 13.77 kg, and the performance factor is 1.80. The paper also analyzes the economic benefits of the system, which aims to improve the desalination speed and rate of seawater, and enhance the wind power generation capacity. | |
| − | '''Keywords | + | '''Keywords''': Peak carbon dioxide emissions, carbon neutrality, seawater desalination, natural vacuum distillation, economic viability |
| − | + | ==1. Introduction== | |
| − | + | Climate change is a global issue that poses a serious threat to the biosphere, as many countries persistently emit CO2 and cause a sharp increase in greenhouse gases. In September 2020, General Secretary Xi Jinping announced China's ambition to peak its carbon dioxide emissions before 2030 and achieve carbon neutrality by 2060 [1]. This is not only a prerequisite for China's sustainable and high-quality development, but also a top priority in building a community of shared future for mankind. Desalination, as an open water supply technology, can augment China’s total freshwater resources and alleviate coastal water scarcity. However, it entails energy consumption during operation and construction, which necessitates the reduction of carbon emissions, the improvement of energy efficiency, and the enhancement of desalination equipment quality to effectively promote industrial standardization. Therefore, research on desalination technology is of paramount importance. The “Outline of the Fourteenth Five-Year Plan for National Economic and Social Development and the Long-Range Objectives Through the Year 2035 of the People's Republic of China” highlights the need to “promote the large-scale development of desalination and ocean energy,” which is one of the greatest challenges China currently faces [2]. In 2021, with the successive release of policy documents on “peaking carbon dioxide emissions” and “achieving carbon neutrality” by the central government and the State Council, changes in China's energy structure, along with improved energy utilization efficiency, will present new opportunities and challenges for China's desalination industry. | |
| − | + | ||
| − | Climate change is a global issue that poses a serious threat to the biosphere, as many countries persistently emit CO2 and cause a sharp increase in greenhouse gases. In September 2020, General Secretary Xi Jinping announced China's ambition to peak its carbon dioxide emissions before 2030 and achieve carbon neutrality by 2060 [1]. This is not only a prerequisite for China's sustainable and high-quality development, but also a top priority in building a community of shared future for mankind. Desalination, as an open water supply technology, can augment China’s total freshwater resources and alleviate coastal water scarcity. However, it entails energy consumption during operation and construction, which necessitates the reduction of carbon emissions, the improvement of energy efficiency, and the enhancement of desalination equipment quality to effectively promote industrial standardization. Therefore, research on desalination technology is of paramount importance. The “Outline of the Fourteenth Five-Year Plan for National Economic and Social Development and the Long-Range Objectives Through the Year 2035 of the People's Republic of China” highlights the need to “promote the large-scale development of desalination and ocean energy,” which is one of the greatest challenges China currently faces [2]. In 2021, with the successive release of policy documents on “peaking carbon dioxide emissions” and “achieving carbon neutrality” by the central government and the State Council, | + | |
Two main methods are adopted by the international community for seawater desalination: thermal methods (multistage flash distillation and low-temperature multiple effect distillation) and membrane methods (reverse osmosis). However, both methods require a certain amount of energy to substitute existing freshwater resources, and the use of conventional energy sources inevitably results in CO2 emissions. Thermal desalination involves both power generation and thermal desalination, while reverse osmosis desalination does not involve phase changes, leading to lower energy consumption and only requiring electricity. The electricity consumption per ton of water is approximately 3-4 kWh. Freshwater resources in coastal, arid, and semi-arid regions in China have become the primary limiting factor for our economic and environmental development. Seawater desalination technology can effectively address this problem in coastal areas, but its development is hindered by high energy consumption and environmental pollution. The use of new energy sources for seawater desalination is an effective way to tackle high energy consumption and pollution. Coastal areas with wind and solar energy resources are ideal locations for seawater desalination. Aznar-Sánchez et al. and others have studied the low-temperature multiple effect distillation seawater desalination process, and found that although this method has very low energy consumption, it still consumes significant energy, and they have pointed out that the water quality from membrane treatment is inferior to that of thermal methods [3]. Guo et al. briefly introduced the situation of seawater desalination worldwide and pointed out that the main challenges currently are excessive energy consumption and significant room for development [4]. Vacuum distillation can achieve seawater desalination, but maintaining the vacuum level requires a large amount of electricity. As the vacuum level increases, both the vacuum rate and power consumption will increase significantly. Choi et al. utilized natural vacuum distillation technology, that is, under the interaction of seawater gravity and atmospheric pressure, to achieve seawater evaporation at room temperature, thereby reducing the use of vacuum pumps [5]. They theoretically studied the desalination process of natural vacuum distillation, and found that evaporation occurs when the seawater pressure is not greater than 4 kPa. They constructed a physical model of this method, and proposed that the technology requires a heat source and the installation of a fan to generate convection, thus improving the system efficiency. Although the power consumption of the system is very low, the power consumption of the auxiliary system is relatively high. Muhammad et al. constructed a mathematical model for solar natural vacuum distillation and based on measurement data from Bahrain, found that this method operates most effectively at a temperature difference of around 20°C [6]. | Two main methods are adopted by the international community for seawater desalination: thermal methods (multistage flash distillation and low-temperature multiple effect distillation) and membrane methods (reverse osmosis). However, both methods require a certain amount of energy to substitute existing freshwater resources, and the use of conventional energy sources inevitably results in CO2 emissions. Thermal desalination involves both power generation and thermal desalination, while reverse osmosis desalination does not involve phase changes, leading to lower energy consumption and only requiring electricity. The electricity consumption per ton of water is approximately 3-4 kWh. Freshwater resources in coastal, arid, and semi-arid regions in China have become the primary limiting factor for our economic and environmental development. Seawater desalination technology can effectively address this problem in coastal areas, but its development is hindered by high energy consumption and environmental pollution. The use of new energy sources for seawater desalination is an effective way to tackle high energy consumption and pollution. Coastal areas with wind and solar energy resources are ideal locations for seawater desalination. Aznar-Sánchez et al. and others have studied the low-temperature multiple effect distillation seawater desalination process, and found that although this method has very low energy consumption, it still consumes significant energy, and they have pointed out that the water quality from membrane treatment is inferior to that of thermal methods [3]. Guo et al. briefly introduced the situation of seawater desalination worldwide and pointed out that the main challenges currently are excessive energy consumption and significant room for development [4]. Vacuum distillation can achieve seawater desalination, but maintaining the vacuum level requires a large amount of electricity. As the vacuum level increases, both the vacuum rate and power consumption will increase significantly. Choi et al. utilized natural vacuum distillation technology, that is, under the interaction of seawater gravity and atmospheric pressure, to achieve seawater evaporation at room temperature, thereby reducing the use of vacuum pumps [5]. They theoretically studied the desalination process of natural vacuum distillation, and found that evaporation occurs when the seawater pressure is not greater than 4 kPa. They constructed a physical model of this method, and proposed that the technology requires a heat source and the installation of a fan to generate convection, thus improving the system efficiency. Although the power consumption of the system is very low, the power consumption of the auxiliary system is relatively high. Muhammad et al. constructed a mathematical model for solar natural vacuum distillation and based on measurement data from Bahrain, found that this method operates most effectively at a temperature difference of around 20°C [6]. | ||
| − | Natural vacuum distillation is a type of vacuum environment generated by natural gravity. It requires high performance of the equipment, using electricity as power without consuming too much electrical energy, achieving significant energy savings. To address the above issues, this paper designs a natural vacuum solar seawater desalination device with water filling and exhaust, which only requires a pipe pump to fill the evaporator with water and discharge the non-condensable gas accumulated in it, thus achieving the purpose of re-evacuation. The system | + | Natural vacuum distillation is a type of vacuum environment generated by natural gravity. It requires high performance of the equipment, using electricity as power without consuming too much electrical energy, achieving significant energy savings. To address the above issues, this paper designs a natural vacuum solar seawater desalination device with water filling and exhaust, which only requires a pipe pump to fill the evaporator with water and discharge the non-condensable gas accumulated in it, thus achieving the purpose of re-evacuation. The system, consisting of an evaporator, a condenser, and several pipelines, is characterized by a simple structure, low cost, low maintenance cost, and long service life. It can effectively solve the problem of domestic water use for residents in isolated and remote areas. The implementation of this project has great scientific value for breaking through China’s green and low-carbon seawater desalination technology, improving energy efficiency, promoting the development of industrial green and low-carbon technology, and advancing the sustainable development of China’s industry. |
| − | =2 Principle of natural vacuum solar seawater desalination system= | + | ==2. Principle of natural vacuum solar seawater desalination system== |
| − | The working principle of the natural vacuum solar seawater desalination system used in this paper is | + | The working principle of the natural vacuum solar seawater desalination system used in this paper is shown in [[#img-1|Figure 1]]. |
| − | <div class=" | + | <div id='img-1'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:11111111.jpg|600x600px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 1'''. Schematic diagram of the experimental system | ||
| + | |} | ||
| − | |||
| − | |||
| − | The system operates as follows: a water | + | The system operates as follows: a water supply pump injects seawater into the evaporator at a height exceeding 10m, and then the exhaust valve expels the seawater in the evaporator. This creates a vacuum in the evaporator by the principle of atmospheric pressure supporting a 10m water column. Subsequently, the raw seawater is condensed by the condenser, recovering the latent heat of condensation. It then enters the constant temperature water tank, where it is reheated by the solar collector, and finally reaches the evaporator. To achieve convective heat transfer and enhance heat transfer efficiency, cooling water pipes and hot water seawater inlet pipes are installed on both sides of the evaporator. Under negative pressure, the seawater saturation temperature decreases, and the heated seawater rapidly vaporizes in the evaporator and condenses into fresh water in the upper condenser. The condensed fresh water falls into the pool and flows into the fresh water storage tank through the water pipe by gravity. |
Non-condensable gases accumulate in the evaporator during the long-term operation of the device, increasing the pressure inside the evaporator and decreasing the evaporation rate. At this point, water replenishment to the evaporator is required, followed by the next cycle. Specifically, the seawater valve leading to the heat exchanger is closed, and then it is introduced into the evaporator, followed by closing the valve leading to the fresh concentrated seawater, allowing it to continue filling with waste gas, and then releasing the non-condensable gas by approaching a vacuum. If no gas is discharged from the pipeline, the valve of the concentrator hose is removed, and the system returns to a vacuum. The experimental equipment is affected by “twist damage,” which lowers the pressure in the evaporator, thereby reducing the vaporization temperature of seawater in the evaporator, enhancing the evaporation effect of low-temperature seawater, and increasing the fresh water output [7]. | Non-condensable gases accumulate in the evaporator during the long-term operation of the device, increasing the pressure inside the evaporator and decreasing the evaporation rate. At this point, water replenishment to the evaporator is required, followed by the next cycle. Specifically, the seawater valve leading to the heat exchanger is closed, and then it is introduced into the evaporator, followed by closing the valve leading to the fresh concentrated seawater, allowing it to continue filling with waste gas, and then releasing the non-condensable gas by approaching a vacuum. If no gas is discharged from the pipeline, the valve of the concentrator hose is removed, and the system returns to a vacuum. The experimental equipment is affected by “twist damage,” which lowers the pressure in the evaporator, thereby reducing the vaporization temperature of seawater in the evaporator, enhancing the evaporation effect of low-temperature seawater, and increasing the fresh water output [7]. | ||
| Line 51: | Line 53: | ||
The experimental apparatus comprises a stainless steel tank as the evaporator, with a diameter of 350mm and a length of 2000mm, and an outer wall covered by a 20mm rubber insulation layer. The condenser is a stainless steel corrugated tube with a diameter of 25mm and a length of 11m, arranged in a serpentine coil configuration within the evaporator to ensure complete and uniform coverage, with a configuration area of 240mm×1900mm. The water collection area has a V-shaped groove with an included angle of 130 degrees, with a 5-degree tilt to the left and right, facilitating the flow of fresh water within the collection area towards the outlet. A 300W electric heating rod substitutes for traditional solar collectors, with temperature control of the hot water tank achieved using a temperature control box. | The experimental apparatus comprises a stainless steel tank as the evaporator, with a diameter of 350mm and a length of 2000mm, and an outer wall covered by a 20mm rubber insulation layer. The condenser is a stainless steel corrugated tube with a diameter of 25mm and a length of 11m, arranged in a serpentine coil configuration within the evaporator to ensure complete and uniform coverage, with a configuration area of 240mm×1900mm. The water collection area has a V-shaped groove with an included angle of 130 degrees, with a 5-degree tilt to the left and right, facilitating the flow of fresh water within the collection area towards the outlet. A 300W electric heating rod substitutes for traditional solar collectors, with temperature control of the hot water tank achieved using a temperature control box. | ||
| − | The project plans to set up three temperature measurement points at equal distances on the top surface of the evaporator, and to set up temperature measurement points at the inlet of cold seawater, the outlet of concentrated brine, and the outlet to accurately predict the water production performance of the system under different operating conditions. Based on this, the temperature data accuracy of the 32-channel rapid temperature recorder (JLS XMT) is ±0.1℃. A vacuum gauge measures the pressure inside the evaporator, with a measurement range from 0 to 100 kPa and an accuracy of ±1.6%. An electronic scale is installed below the water storage tank, with a measurement accuracy of ±0.5g, to record the water quantity in the system in real time [8]. The temperature of the thermocouple and the consumption of clean water are tested for 1 min and 5 min, respectively, before changing the test formula. If the water quantity in the system is less than 0.1 kg/5min, the end of the system’s operating cycle is determined, and a new round of water injection begins.. | + | The project plans to set up three temperature measurement points at equal distances on the top surface of the evaporator, and to set up temperature measurement points at the inlet of cold seawater, the outlet of concentrated brine, and the outlet to accurately predict the water production performance of the system under different operating conditions. Based on this, the temperature data accuracy of the 32-channel rapid temperature recorder (JLS XMT) is ±0.1℃. A vacuum gauge measures the pressure inside the evaporator, with a measurement range from 0 to 100 kPa and an accuracy of ±1.6%. An electronic scale is installed below the water storage tank, with a measurement accuracy of ±0.5g, to record the water quantity in the system in real time [8]. The temperature of the thermocouple and the consumption of clean water are tested for 1 min and 5 min, respectively, before changing the test formula. If the water quantity in the system is less than 0.1 kg/5min, the end of the system’s operating cycle is determined, and a new round of water injection begins. The schematic diagram is shown in [[#img-2|Figure 2]]. |
| − | <div class=" | + | <div id='img-2'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:222222.png|600x600px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 2'''. Temperature measurement point layout | ||
| + | |} | ||
| − | |||
| − | |||
| − | =3 Performance analysis= | + | ==3. Performance analysis== |
| − | ==3.1 Energy analysis of the system== | + | ===3.1 Energy analysis of the system=== |
The system’s heat transfer and water production were studied by further analyzing the heat change process and freshwater production process in the system based on the energy conservation equation. In this paper, we assume that the evaporator has good adiabatic characteristics and neglect external heat exchange. | The system’s heat transfer and water production were studied by further analyzing the heat change process and freshwater production process in the system based on the energy conservation equation. In this paper, we assume that the evaporator has good adiabatic characteristics and neglect external heat exchange. | ||
| − | In the evaporator, hot seawater is continuously evaporated, and the energy consumed is the heat of vaporization carried away by the water vapor, as shown specifically in | + | In the evaporator, hot seawater is continuously evaporated, and the energy consumed is the heat of vaporization carried away by the water vapor, as shown specifically in Eq. (1): |
| − | <math>\phi_h=q_{m_w}c_{\rho_w}t_6-q_{m_b\rho_w}t_7</math> | + | {| class="formulaSCP" style="width: 100%; text-align: left;" |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>\phi_h=q_{m_w}c_{\rho_w}t_6-q_{m_b\rho_w}t_7</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(1) | ||
| + | |} | ||
| − | + | where <math>q_{m_w}</math> represents the mass flow rate of seawater entering the evaporator at a speed of kg/s, <math>q_{m_{\mathfrak{b}}}</math> the mass flow rate of concentrated seawater discharged from the evaporator at a speed of kg/s, <math>\mathcal{C}_{\rho_{\mathrm{w}}}</math> the specific heat capacity of seawater at constant pressure in units of kj/(kg.k), <math>t_{6}</math> the water temperature of seawater entering the evaporator in units of ℃, and <math>t_{7}</math> the water temperature of concentrated seawater discharged from the evaporator in units of ℃. | |
| − | When seawater is in contact with fresh water and low-temperature condensers in the hot sea, due to the increase in vapor content, the phenomenon of fresh water condensation occurs, and the flow rate of fresh water produced by the device is as shown in | + | When seawater is in contact with fresh water and low-temperature condensers in the hot sea, due to the increase in vapor content, the phenomenon of fresh water condensation occurs, and the flow rate of fresh water produced by the device is as shown in Eq. (2): |
| − | + | {| class="formulaSCP" style="width: 100%; text-align: left;" | |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>q_{m_f}=\frac{\phi_h}{h_{fg}}</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(2) | ||
| + | |} | ||
| − | <math>h_{fg} | + | where <math>h_{fg}</math> represents the latent heat at different condensation temperatures, obtained empirically as Eq. (3): |
| − | + | {| class="formulaSCP" style="width: 100%; text-align: left;" | |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>h_{fg}=2501.897-2.407t_w+1.192\times 10^{-3}t^2_w-1.586\times 10^{-3}t^3_w</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(3) | ||
| + | |} | ||
| − | + | where <math>t_{w}</math> represents the condensation temperature of water in degrees Celsius. | |
| − | + | When cold seawater flows through the condenser, it enhances the system’s energy efficiency by absorbing the condensation latent heat from the cold water passing through the condenser. The heat gained during this process is represented by Eq. (4): | |
| − | + | {| class="formulaSCP" style="width: 100%; text-align: left;" | |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>\phi_c=q_{m_w}c_{\rho_w}\left(t_{5-}t_{c,in}\right)</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(4) | ||
| + | |} | ||
| − | + | where <math>t_{5}</math> represents the temperature of cold seawater flowing out of the condenser in degrees Celsius, and <math>t_{c}</math> the temperature of cold seawater flowing into the condenser in degrees Celsius. | |
| − | + | ===3.2 Water quality analysis=== | |
| − | + | Seawater desalination can be achieved by two methods: thermal and membrane. The system used in this project is based on the thermal method, which produces freshwater with lower salt content compared to the membrane method. Moreover, it operates at room temperature, unlike conventional thermal techniques. Compared to traditional thermal techniques, the salt molecules have smaller, milder, and higher entropy distributions, resulting in lower salt content in the freshwater [9]. [[#tab-1|Table 1]] shows the water quality of each conventional process, demonstrating that the salt content in the water treated by thermal processes is extremely low. Therefore, the salt content in the factory’s effluent will be reduced to below 5mg/L. | |
| − | Table 1 | + | |
| − | + | <div class="center" style="font-size: 85%;">'''Table 1'''. Water quality of traditional technologies</div> | |
| − | + | ||
| − | | | + | <div id='tab-1'></div> |
| − | + | {| class="wikitable" style="margin: 1em auto 0.1em auto;border-collapse: collapse;font-size:85%;width:auto;" | |
| − | | | + | |-style="text-align:center" |
| − | |- | + | !Traditional seawater desalination technology !! Method !! Salinity (mg·L<sup>-1</sup>) |
| − | + | |-style="text-align:center" | |
| − | | | + | | Low-temperature multiple effect |
| − | | | + | | Thermal method |
| − | |- | + | | 5 |
| − | + | |-style="text-align:center" | |
| − | | | + | | Multi-stage flash distillation |
| − | | | + | | Thermal method |
| − | |- | + | | 5 |
| − | + | |-style="text-align:center" | |
| − | | | + | | Vapor compression distillation |
| − | | | + | | Thermal method |
| − | |- | + | | 5 |
| − | + | |-style="text-align:center" | |
| − | | | + | | Reverse osmosis |
| − | | | + | | Membrane method |
| + | | 500 | ||
|} | |} | ||
| − | |||
| − | ==4.1 Feasibility verification test of the device== | + | ==4. Experimental results and discussion== |
| + | |||
| + | ===4.1 Feasibility verification test of the device=== | ||
Using a wind turbine model with a 150 W electric motor and a simulated vacuum pump, the temperature of unheated fresh water at 25°C was significantly reduced to 25°C after heating to the boiling point, while the temperature of unheated seawater at 25°C was noticeably reduced to 45°C after heating to the boiling point (the temperature indicator of fresh water after heating to the boiling point was unstable, with a large amount of dissolved gas being released). | Using a wind turbine model with a 150 W electric motor and a simulated vacuum pump, the temperature of unheated fresh water at 25°C was significantly reduced to 25°C after heating to the boiling point, while the temperature of unheated seawater at 25°C was noticeably reduced to 45°C after heating to the boiling point (the temperature indicator of fresh water after heating to the boiling point was unstable, with a large amount of dissolved gas being released). | ||
| − | + | To investigate the feasibility of the equipment’s working principle, we conducted a series of evaporation experiments using fresh water and seawater at different temperatures. We measured the number of bubbles formed during the evaporation process as an indicator of the amount of dissolved air and the evaporation rate. As depicted in [[#tab-2|Table 2]], we found that fresh water that was heated to boiling and then cooled to 25°C had fewer bubbles than fresh water that was unheated at 25°C, suggesting that heating had removed most of the dissolved air from the water. Dissolved air was more volatile than fresh water and thus evaporated faster. We also observed that seawater at 25°C had fewer bubbles than fresh water at 25°C, indicating that seawater had a lower evaporation rate than fresh water. This could be due to the higher salinity and density of seawater, which reduced its vapour pressure. Furthermore, we compared the temperature of fresh water that was heated to boiling and then cooled to 25°C and 45°C, respectively. We found that the higher temperature increased the evaporation rate, as evidenced by the more rapid decrease in water volume before and after boiling. These results confirmed the validity of the equipment’s working principle. | |
| − | Table 2 | + | |
| − | {| style=" | + | <div class="center" style="font-size: 85%;">'''Table 2'''. Device verification test data</div> |
| + | |||
| + | <div id='tab-2'></div> | ||
| + | {| class="wikitable" style="margin: 1em auto 0.1em auto;border-collapse: collapse;font-size:85%;width:auto;" | ||
| + | |-style="text-align:center" | ||
| + | ! Vaporization experiment !! Initial observations !! Observations after 15 minutes | ||
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Untreated fresh water at 25℃ |
| − | + | | style="text-align: center;vertical-align: top;"|Numerous bubbles with diameters of 3.5cm and 1cm | |
| − | + | | style="text-align: center;vertical-align: top;"|Decrease in the initial bubble quantity | |
| − | + | ||
| − | + | ||
| − | | style=" | + | |
| − | | style=" | + | |
|- | |- | ||
| style="text-align: center;vertical-align: top;"|Fresh water heated to boiling and then cooled to 25℃ | | style="text-align: center;vertical-align: top;"|Fresh water heated to boiling and then cooled to 25℃ | ||
| Line 142: | Line 180: | ||
| style="text-align: center;vertical-align: top;"|Decrease in the initial bubble quantity | | style="text-align: center;vertical-align: top;"|Decrease in the initial bubble quantity | ||
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Seawater heated to boiling and then cooled to 25℃ |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Vigorous boiling |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Almost no change in the initial bubble quantity |
|} | |} | ||
| − | + | ===4.2 Desalination experiment simulation=== | |
| − | + | Seawater evaporation under different wind speeds at room temperature (25°C) was investigated using a cylindrical evaporator. The wind speeds were varied incrementally from the previous limit, and the experimental results are presented in [[#img-3|Figure 3]]. As shown in [[#img-3|Figure 3]], the evaporation rate of seawater increased with the wind speed, matching the pumping rate of the vacuum pump, which resulted in a decrease and stabilization of the air pressure at a certain value. The time required to reach the maximum pressure for each wind speed was shorter than that for the original pressure, as the seawater evaporated faster at lower pressure, replenishing the air pressure more rapidly. The gas pressure reached a steady state in a shorter time span as the evaporation rate increased. | |
| − | + | <div id='img-3'></div> | |
| + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | ||
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:333333.png|500px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 3'''. Relationship between air pressure and wind speed | ||
| + | |} | ||
| − | |||
| − | |||
| − | + | The boiling point of seawater varies depending on its composition and concentration, which differ across regions, and there is no standard reference for it. Therefore, we used the boiling point of fresh water at the corresponding limit pressure as a reference to estimate the critical pressure values obtained at different velocities, as shown in [[#img-4|Figure 4]]. The critical pressure decreased and stabilized as the velocity increased. The boiling point of fresh water also decreased gradually, indicating that the same trend would apply to seawater. | |
| − | Figure | + | |
| − | + | <div id='img-4'></div> | |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| − | <div class=" | + | |-style="background:white;" |
| − | + | |style="text-align: center;padding:10px;"| [[File:444444.png|500px]] | |
| − | + | |- | |
| − | + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 4'''. Boiling point of fresh water at the corresponding wind speed and corresponding air pressure limit pressure | |
| − | Figure 4 Boiling point of fresh water at the corresponding wind speed and | + | |} |
| − | |||
| − | |||
The experimental phenomena and data presented above lead to the following conclusions: the critical pressure is inversely proportional to the wind speed and the evaporation rate is directly proportional to it; both fresh water and seawater exhibit abundant bubbling in the initial stage of evaporation due to the release of gases during the process; the evaporation rate of seawater can be significantly enhanced by heating; the evaporation rate also depends on the type of liquid, resulting in different maximum pressures that can be achieved by various liquids in the cylindrical evaporation chamber under the same flow rate; a rotary vane vacuum pump was chosen as the equipment model, which has a relatively small pumping chamber, requiring a high rotational speed to increase the pumping speed. In the experiment, the wind power was sufficient, exceeding 150 W, but the wind turbine had a corresponding speed for each wind speed. However, the rotational speed of the wind turbine was still insufficient for the vacuum pump, and a lot of wind energy was wasted, making it difficult to lower the limiting pressure and to evaporate seawater at room temperature. Therefore, the suggested improvement for this model is to select a larger vacuum pump or to install larger gears on the original vacuum pump to better choose the type and size of the blades, and to design the cylindrical steam chamber size rationally. | The experimental phenomena and data presented above lead to the following conclusions: the critical pressure is inversely proportional to the wind speed and the evaporation rate is directly proportional to it; both fresh water and seawater exhibit abundant bubbling in the initial stage of evaporation due to the release of gases during the process; the evaporation rate of seawater can be significantly enhanced by heating; the evaporation rate also depends on the type of liquid, resulting in different maximum pressures that can be achieved by various liquids in the cylindrical evaporation chamber under the same flow rate; a rotary vane vacuum pump was chosen as the equipment model, which has a relatively small pumping chamber, requiring a high rotational speed to increase the pumping speed. In the experiment, the wind power was sufficient, exceeding 150 W, but the wind turbine had a corresponding speed for each wind speed. However, the rotational speed of the wind turbine was still insufficient for the vacuum pump, and a lot of wind energy was wasted, making it difficult to lower the limiting pressure and to evaporate seawater at room temperature. Therefore, the suggested improvement for this model is to select a larger vacuum pump or to install larger gears on the original vacuum pump to better choose the type and size of the blades, and to design the cylindrical steam chamber size rationally. | ||
| − | ==4.3 The system’s water production rate under continuous cycling conditions== | + | ===4.3 The system’s water production rate under continuous cycling conditions=== |
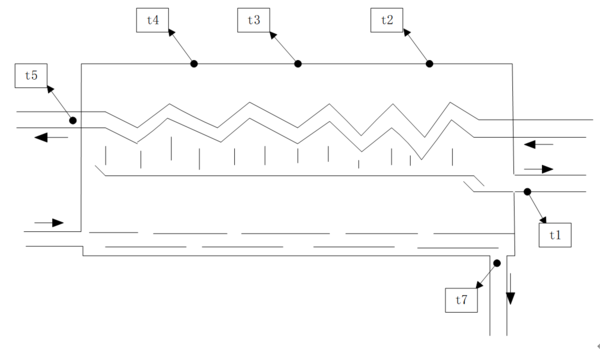
| − | The above experiment used experimental seawater as the raw material and applied a constant temperature control to the hot box using a temperature control box. The preparation process involved inflating and draining the evaporator for about 20 minutes to establish a vacuum inside. After the discharge was completed, the evaporator could generate an internal absolute pressure of about 8 kPa. Figure 5 shows the temperature change of the top outer wall of the evaporator with the temperature, with seawater heated to 50°C and a mass flow rate of 0.1 kg/s. The experimental results indicate that the temperature of the outer wall of the evaporator first increases sharply, and then decreases sharply during the second filling process, exhibiting a complete cycle process [10]. When the heated seawater is injected into the evaporator, it undergoes instant boiling when it reaches the saturation temperature corresponding to its internal pressure, with some of the steam being condensed into fresh water in the condenser, while the remaining hot steam and non-condensing gas accumulate at the top of the evaporator under the influence of buoyancy, thereby increasing the temperature and pressure inside the evaporator. It can be observed that the temperature of the evaporator wall ( | + | The above experiment used experimental seawater as the raw material and applied a constant temperature control to the hot box using a temperature control box. The preparation process involved inflating and draining the evaporator for about 20 minutes to establish a vacuum inside. After the discharge was completed, the evaporator could generate an internal absolute pressure of about 8 kPa. [[#img-5|Figure 5]] shows the temperature change of the top outer wall of the evaporator with the temperature, with seawater heated to 50°C and a mass flow rate of 0.1 kg/s. The experimental results indicate that the temperature of the outer wall of the evaporator first increases sharply, and then decreases sharply during the second filling process, exhibiting a complete cycle process [10]. When the heated seawater is injected into the evaporator, it undergoes instant boiling when it reaches the saturation temperature corresponding to its internal pressure, with some of the steam being condensed into fresh water in the condenser, while the remaining hot steam and non-condensing gas accumulate at the top of the evaporator under the influence of buoyancy, thereby increasing the temperature and pressure inside the evaporator. It can be observed that the temperature of the evaporator wall (<math display="inline"> t_2</math>) is lower near the cold water inlet, indicating higher cooling efficiency, as cold water differs from hot water. |
| − | <div class=" | + | <div id='img-5'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:555555.png|500px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 5'''. Variation of the temperature at the top of the evaporator | ||
| + | |} | ||
| − | |||
| − | |||
| − | Figure 6 illustrates the relationship between the outlet temperature of each pipeline and time. It can be seen from Figure 6 that the outlet temperature | + | [[#img-6|Figure 6]] illustrates the relationship between the outlet temperature of each pipeline and time. It can be seen from [[#img-6|Figure 6]] that the outlet temperature <math display="inline"> t_7</math> of concentrated seawater increases with time, indicating that the evaporation rate of high-temperature seawater continues to decrease. Consequently, at the end of the cycle, <math display="inline"> t_7</math> has risen to 47℃. The outlet temperature <math display="inline"> t_5</math> of cold seawater sharply rises and then gradually decreases because when the hot water enters the low-pressure evaporator, it evaporates rapidly. The vapor is significant, and when it comes into contact with the condenser, latent heat is rapidly released, condensing into fresh water, causing the temperature <math display="inline"> t_5</math> to rise rapidly. The smaller the evaporation coefficient, the less heat transfer occurs between the steam and the condensing tube, resulting in a smaller <math display="inline"> t_5</math>. Additionally, since the temperature of the fresh water from condensation is mainly influenced by the temperature of the condensing tube, the trend of the water outlet temperature <math display="inline"> t_1</math> is similar to the trend of <math display="inline"> t_5</math> but with a much smaller magnitude of change. |
| − | <div class=" | + | <div id='img-6'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:666666.png|500px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 6'''. Variation of outlet temperatures for each pipeline | ||
| + | |} | ||
| − | |||
| − | |||
| − | The pressure and instantaneous water production of the two cycles inside the evaporator were theoretically calculated and the results were consistent with the experimental results. Based on this, a new design scheme was proposed according to the experimental results, adopting a new design. The pressure in the distillation tower increased rapidly at the beginning of heating the water, and the yield was also high, producing 1.05 kg of fresh water in 5 minutes. However, as the pressure increased, the water production rate gradually decreased. This was mainly attributed to the fact that in the distillation device, the overflow speed of steam and non-condensable gas was very fast when the steam was at very low pressure. When the steam was condensed into fresh water, the accumulation of steam in the distillation device increased. During this process, the non-condensable gas hindered the condensation heat transfer of water vapor in the condensation tube, causing a large amount of water vapor to accumulate in the evaporator, resulting in an increase in pressure and a decrease in water production. As shown in Figure 4, at a temperature of 50°C, when the hot seawater entered the equipment, the low temperature was severely affected by the non-condensable gas, so after excluding the time for filling and draining water, each cycle could only operate for 35 minutes, which was not conducive to the equipment operation. | + | The pressure and instantaneous water production of the two cycles inside the evaporator were theoretically calculated and the results were consistent with the experimental results. Based on this, a new design scheme was proposed according to the experimental results, adopting a new design. The pressure in the distillation tower increased rapidly at the beginning of heating the water, and the yield was also high, producing 1.05 kg of fresh water in 5 minutes. However, as the pressure increased, the water production rate gradually decreased. This was mainly attributed to the fact that in the distillation device, the overflow speed of steam and non-condensable gas was very fast when the steam was at very low pressure. When the steam was condensed into fresh water, the accumulation of steam in the distillation device increased. During this process, the non-condensable gas hindered the condensation heat transfer of water vapor in the condensation tube, causing a large amount of water vapor to accumulate in the evaporator, resulting in an increase in pressure and a decrease in water production. As shown in [[#img-4|Figure 4]], at a temperature of 50°C, when the hot seawater entered the equipment, the low temperature was severely affected by the non-condensable gas, so after excluding the time for filling and draining water, each cycle could only operate for 35 minutes, which was not conducive to the equipment operation. |
| − | ==4.4 Comparison of water production performance at different hot sea water temperatures== | + | ===4.4 Comparison of water production performance at different hot sea water temperatures=== |
| − | Different temperatures of warm sea water will have a significant impact on the system’s operational capacity. Under the condition of constant water supply of 0.1 kg/s, the relationship between the internal pressure of the evaporator and the high-temperature sea water is shown in Figure 7. | + | Different temperatures of warm sea water will have a significant impact on the system’s operational capacity. Under the condition of constant water supply of 0.1 kg/s, the relationship between the internal pressure of the evaporator and the high-temperature sea water is shown in [[#img-7|Figure 7]]. |
| − | <div class=" | + | <div id='img-7'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:777777.png|500px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 7'''. Pressure at different hot sea water temperatures | ||
| + | |} | ||
| − | |||
| − | |||
| − | Figure 7 illustrates that the working cycle time of the system and the degree of pressure increase both decrease as the temperature of the hot sea water decreases. This is because the reaction with non-condensing gas is faster at lower water temperatures. Due to the lower temperature, less sea water evaporates, and although the emission of non-condensing gas is also small, its proportion of the vapor is large, thus shortening the operation time [11]. Figure 8 shows the cumulative water production of the system corresponding to different water temperatures. The cumulative water production of the system increases with the increase of water temperature. Under the condition of water temperature of 60°C, the system has a working cycle of 75 minutes, and a cumulative water production of 13.77 kg, which means it produces 10.98 kg of fresh water per hour. | + | [[#img-7|Figure 7]] illustrates that the working cycle time of the system and the degree of pressure increase both decrease as the temperature of the hot sea water decreases. This is because the reaction with non-condensing gas is faster at lower water temperatures. Due to the lower temperature, less sea water evaporates, and although the emission of non-condensing gas is also small, its proportion of the vapor is large, thus shortening the operation time [11]. [[#img-8|Figure 8]] shows the cumulative water production of the system corresponding to different water temperatures. The cumulative water production of the system increases with the increase of water temperature. Under the condition of water temperature of 60°C, the system has a working cycle of 75 minutes, and a cumulative water production of 13.77 kg, which means it produces 10.98 kg of fresh water per hour. |
| − | <div class=" | + | <div id='img-8'></div> |
| − | + | {| class="wikitable" style="margin: 0em auto 0.1em auto;border-collapse: collapse;width:auto;" | |
| + | |-style="background:white;" | ||
| + | |style="text-align: center;padding:10px;"| [[File:888888.png|500px]] | ||
| + | |- | ||
| + | | style="background:#efefef;text-align:left;padding:10px;font-size: 85%;"| '''Figure 8'''. Cumulative water production at different hot seawater temperatures | ||
| + | |} | ||
| − | |||
| − | |||
| − | The performance coefficient (GOR) expresses the performance evaluation index of the system. According to the energy cost/production ratio, the equipment efficiency coefficient is the ratio of the latent heat required for freshwater production, which can be calculated using | + | The performance coefficient (GOR) expresses the performance evaluation index of the system. According to the energy cost/production ratio, the equipment efficiency coefficient is the ratio of the latent heat required for freshwater production, which can be calculated using Eq. (5): |
| − | <math>COR=\frac{h_{fg}q_{mf}}{q_{m_w}c_{\rho_w}(t_6-t_5)}</math> | + | {| class="formulaSCP" style="width: 100%; text-align: left;" |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>COR=\frac{h_{fg}q_{mf}}{q_{m_w}c_{\rho_w}(t_6-t_5)}</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(5) | ||
| + | |} | ||
| − | According to | + | According to Eq. (5), the performance coefficient of the system at different water temperatures is shown in [[#tab-3|Table 3]]. From [[#tab-3|Table 3]], it can be seen that the heat transfer coefficient increases with the rise of water temperature, and the heat transfer coefficient can be as high as 1.80. This is because as the water temperature increases, the heat transfer between steam and condenser increases, thereby improving the condensation efficiency. Improving the fresh water efficiency can allow cold water to absorb more evaporative potential, thereby improving energy efficiency. |
| − | <div class="center" style=" | + | <div class="center" style="font-size: 85%;">'''Table 3'''. Compare the GOR at different water temperatureS</div> |
| − | Table | + | |
| − | {| | + | <div id='tab-3'></div> |
| − | + | {| class="wikitable" style="margin: 1em auto 0.1em auto;border-collapse: collapse;font-size:85%;width:auto;" | |
| − | + | |-style="text-align:center" | |
| − | | | + | ! Temperature of hot spring water (°C) !! Amount of water produced per cycle (kg) !! Cycle time /min !! GOR |
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|45 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|1.03 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|40 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|1.62 |
|- | |- | ||
| style="text-align: center;vertical-align: top;"|50 | | style="text-align: center;vertical-align: top;"|50 | ||
| Line 242: | Line 298: | ||
| style="text-align: center;vertical-align: top;"|1.72 | | style="text-align: center;vertical-align: top;"|1.72 | ||
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|60 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|13.77 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|75 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|1.80 |
|} | |} | ||
| − | =5 Economic analysis of the system= | + | ==5. Economic analysis of the system== |
| − | As a solar-powered seawater desalination device, its economic performance directly affects whether it can be commercialized and widely promoted, so the study of its economic performance is particularly important. Here, as represented by | + | As a solar-powered seawater desalination device, its economic performance directly affects whether it can be commercialized and widely promoted, so the study of its economic performance is particularly important. Here, as represented by Eqs. (6) to (7), total cost (<math display="inline">C_{TC}</math>) consists of fixed costs (<math display="inline">C_{FC}</math>) and variable costs (<math display="inline">C_{VC}</math>). Fixed costs are the purchase and installation costs of each device, while variable costs are maintenance costs caused by system losses: |
| − | <math>C_{TC}=C_{FC}+C_{VC}</math> | + | {| class="formulaSCP" style="width: 100%; text-align: left;" |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>C_{TC}=C_{FC}+C_{VC}</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(6) | ||
| + | |} | ||
| − | <math>C_{VC}=s\times N\times C_{FC}</math> | + | {| class="formulaSCP" style="width: 100%; text-align: left;" |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>C_{VC}=s\times N\times C_{FC}</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(7) | ||
| + | |} | ||
| − | + | where <math display="inline"> s </math> represents the annual loss factor of the system, including the losses of various components such as pumps, evaporators, etc., and <math display="inline">N </math> represents the effective lifespan of the system, denoted as '<math display="inline"> a </math>'. By using Eq. (8), calculate the number of flat solar panels required for the system: | |
| − | <math> | + | {| class="formulaSCP" style="width: 100%; text-align: left;" |
| + | |- | ||
| + | | | ||
| + | {| style="text-align: center; margin:auto;width: 100%;" | ||
| + | |- | ||
| + | | style="text-align: center;" |<math>A_c n l_{a\nu}\eta_c = q_{m_w}c_{\rho_w}(t_6-t_5)</math> | ||
| + | |} | ||
| + | | style="width: 5px;text-align: right;white-space: nowrap;" |(8) | ||
| + | |} | ||
| − | + | where <math display="inline"> A_c </math> represents the area of each panel, <math display="inline"> A_c=2</math> m<sup>2</sup>, <math display="inline"> n </math> is the number of different types of flat plate collectors, <math display="inline">l_{a\nu}</math> represents the average intensity of sunlight, <math display="inline"> w </math>, and <math>\eta_{c}</math> represents the average efficiency of solar cells, which is 0.5% per day. | |
| − | If the required system can produce 10.98kg of fresh water per hour, it means that under the conditions of a flow rate of 0.1kg/s, a hot seawater temperature of 60°C, and an average daily solar radiation intensity of 850w/ | + | If the required system can produce 10.98kg of fresh water per hour, it means that under the conditions of a flow rate of 0.1kg/s, a hot seawater temperature of 60°C, and an average daily solar radiation intensity of 850w/m<sup>2</sup>, the number of solar collectors required can be calculated as 6 using formula (8). The construction costs of each system component are listed in [[#tab-4|Table 4]], and the total fixed costs calculated in the following manner are <math>C_{FC}=4000</math> yuan/ton. Due to the fewer system components and the scarcity of system elements and parts, it is assumed that the annual <math display="inline">C_{VC}</math> value is 10% of <math display="inline">C_{FC}</math>, i.e., S=0.1. |
| − | <div class="center" style=" | + | <div class="center" style="font-size: 85%;">'''Table 4'''. Cost of each component in the system</div> |
| − | Table | + | |
| − | + | <div id='tab-1'></div> | |
| − | | | + | {| class="wikitable" style="margin: 1em auto 0.1em auto;border-collapse: collapse;font-size:85%;width:auto;" |
| − | + | |-style="text-align:center" | |
| − | + | ! No. !! Component name !! Fixed cost (yuan/ton) | |
| − | | | + | |
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|1 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Evaporator |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|900 |
|- | |- | ||
| style="text-align: center;vertical-align: top;"|2 | | style="text-align: center;vertical-align: top;"|2 | ||
| Line 282: | Line 360: | ||
|- | |- | ||
| style="text-align: center;vertical-align: top;"|3 | | style="text-align: center;vertical-align: top;"|3 | ||
| − | | style="text-align: center;vertical-align: top;"|Freshwater | + | | style="text-align: center;vertical-align: top;"|Freshwater collection tank |
| style="text-align: center;vertical-align: top;"|100 | | style="text-align: center;vertical-align: top;"|100 | ||
|- | |- | ||
| style="text-align: center;vertical-align: top;"|4 | | style="text-align: center;vertical-align: top;"|4 | ||
| − | | style="text-align: center;vertical-align: top;"|Solar | + | | style="text-align: center;vertical-align: top;"|Solar collector |
| style="text-align: center;vertical-align: top;" |6×350 | | style="text-align: center;vertical-align: top;" |6×350 | ||
|- | |- | ||
| style="text-align: center;vertical-align: top;"|5 | | style="text-align: center;vertical-align: top;"|5 | ||
| − | | style="text-align: center;vertical-align: top;"|Circulating | + | | style="text-align: center;vertical-align: top;"|Circulating water pump |
| style="text-align: center;vertical-align: top;"|120 | | style="text-align: center;vertical-align: top;"|120 | ||
|- | |- | ||
| style="text-align: center;vertical-align: top;"|6 | | style="text-align: center;vertical-align: top;"|6 | ||
| − | | style="text-align: center;vertical-align: top;"|Valves and | + | | style="text-align: center;vertical-align: top;"|Valves and pipelines |
| style="text-align: center;vertical-align: top;"|130 | | style="text-align: center;vertical-align: top;"|130 | ||
|- | |- | ||
| style="text-align: center;vertical-align: top;"|7 | | style="text-align: center;vertical-align: top;"|7 | ||
| − | | style="text-align: center;vertical-align: top;"|Installation | + | | style="text-align: center;vertical-align: top;"|Installation cost |
| style="text-align: center;vertical-align: top;"|500 | | style="text-align: center;vertical-align: top;"|500 | ||
|- | |- | ||
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|8 |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|Total fixed cost |
| − | | style=" | + | | style="text-align: center;vertical-align: top;"|4000 |
|} | |} | ||
| − | Conservatively estimating the service life of the system to be 18 years, with <math>C_{TC}=4000+0.1\times(18-1)\ | + | Conservatively estimating the service life of the system to be 18 years, with <math>C_{TC}=4000+0.1\times(18-1)\times 4000=10800</math> yuan/ton, the city experiences over 300 clear days per year, allowing for continuous operation for 8 hours a day. The daily production of fresh water is <math display="inline">10.98\times 8=87.84</math>kg, resulting in a total fresh water production of 474.34 tons over 18 years. As a result, the average cost of producing one cubic meter of water is 22.5 yuan/ton, which is lower than the conventional solar distillation system (33.6 yuan/ton). Although this cost is relatively high compared to tap water in the city, it is still acceptable when compared to the cost of pure water at 400 yuan/ton. |
| − | =6 Conclusion= | + | ==6. Conclusion== |
| − | This paper presents a solar-powered seawater desalination device with a water-filled exhaust-type natural vacuum distillation system, which can desalinate seawater using solar energy. The seawater enters the device and passes through a condenser first, then enters the solar collector, where the water temperature is raised. The high-temperature seawater boils in the vacuum evaporator, accelerating the evaporation rate. The generated water vapor flows through pipelines and one-way valves into the condenser, where it condenses into fresh water and heats the low-temperature seawater inside the condensation tube. The desalinated water then flows into a freshwater storage tank through pipes. Part of the evaporated seawater is discharged into the sea, while the rest returns to the solar collector through natural convection. The device also uses the water-filling method to discharge non-condensable gases from the device, thereby achieving vacuum restoration. Experimental research has been conducted on this basis, leading to the following conclusions | + | This paper presents a solar-powered seawater desalination device with a water-filled exhaust-type natural vacuum distillation system, which can desalinate seawater using solar energy. The seawater enters the device and passes through a condenser first, then enters the solar collector, where the water temperature is raised. The high-temperature seawater boils in the vacuum evaporator, accelerating the evaporation rate. The generated water vapor flows through pipelines and one-way valves into the condenser, where it condenses into fresh water and heats the low-temperature seawater inside the condensation tube. The desalinated water then flows into a freshwater storage tank through pipes. Part of the evaporated seawater is discharged into the sea, while the rest returns to the solar collector through natural convection. The device also uses the water-filling method to discharge non-condensable gases from the device, thereby achieving vacuum restoration. Experimental research has been conducted on this basis, leading to the following conclusions: |
(1) By using the natural negative pressure principle, the internal absolute pressure of the evaporator can reach 8 kPa. During the system operation, the accumulation of non-condensable gases causes an increase in pressure inside the evaporator. In this situation, a pump can be used to separately inject seawater and fresh water into the evaporator and condenser, and an exhaust valve can be used to discharge non-condensable gases from the evaporator and condenser. Subsequently, the exhaust valve is closed and the drain valve is opened, restoring natural vacuum to the evaporator and condenser, allowing the entire system to continue operating, although the water production rate decreases continuously. | (1) By using the natural negative pressure principle, the internal absolute pressure of the evaporator can reach 8 kPa. During the system operation, the accumulation of non-condensable gases causes an increase in pressure inside the evaporator. In this situation, a pump can be used to separately inject seawater and fresh water into the evaporator and condenser, and an exhaust valve can be used to discharge non-condensable gases from the evaporator and condenser. Subsequently, the exhaust valve is closed and the drain valve is opened, restoring natural vacuum to the evaporator and condenser, allowing the entire system to continue operating, although the water production rate decreases continuously. | ||
| Line 324: | Line 402: | ||
==References== | ==References== | ||
| + | <div class="auto" style="text-align: left;width: auto; margin-left: auto; margin-right: auto;font-size: 85%;"> | ||
| − | [1] J.P. | + | [1] Xi J.P. Speech at the general debate of the 75th session of the United Nations General Assembly. Gov. Gaz. People’s Repub. China, 28:1-3, 2020. |
| − | [2] H. | + | [2] Shi H. Research on carbon footprint accounting method for petroleum and chemical enterprise products. Pet. Petrochem. Mater. Proc., 16:161–162, 2021. |
| − | [3] | + | [3] Aznar-Sánchez A., Belmonte-Urea L.J., Velasco-Muñoz J.F., Valera D.L. Farmers’ profiles and behaviours toward desalinated seawater for irrigation: Insights from South-east Spain. J. Clean. Prod., 296, 126568, 2021. |
| − | [4] C. | + | [4] Guo C., Zhao J., Zhang T. Development of a modified composite structure from polyurethane sponge for desalinating seawater polluted by oil. Desalination, 524, 115471, 2022. |
| − | [5] W.Y. | + | [5] Choi W.Y., Aravena C., Park J., Kang D., Yoo Y. Performance prediction and evaluation of CO2 utilization with conjoined electrolysis and carbonation using desalinated rejected seawater brine. Desalination, 509, 115068, 2021. |
| − | [6] Q. | + | [6] Muhammad Q., Mohamed B., Noor A. Reverse osmosis desalination: A state-of-the-art review. Desalination, 459:59–104, 2019. |
| − | [7] X. | + | [7] Wu X., Liu W., Gao H., Alfaro D., Sun S.R., Lei R.R., Jia T.Q., Zheng M.H. Coordinated effects of air pollution control devices on PAH emissions in coal-fired power plants and industrial boilers. Sci. Total Environ., 756, 144063, 2021. |
| − | [8] S. | + | [8] Wu S., Su L., Ge Y. Assessment of carbon footprint and emission reduction strategies for seawater desalination lifecycle. Ecol. Econ., 261:24–26, 2012. |
| − | [9] Z. | + | [9] Li Z., Zhang D., Pan L. Low-carbon energy transition path and suggestions for China under the “dual carbon” target. J. Power Eng., 41:905–909, 2021. |
| − | [10] L. | + | [10] Zuo L., Zhou T., Li C. Techno-economic analysis of wind-assisted solar chimney power plants with combined seawater desalination and waste heat utilization. Renew. Energy, 40:1459–1464, 2022. |
| − | [11] S. | + | [11] Liu S., Zhang F., Wang J., Chen A., Li L. Research on carbon reduction paths and strategies for seawater desalination in China under the “dual carbon” target. Environ. Sci. Manag., 47:15–19, 2022. |
Latest revision as of 13:32, 15 May 2024
Abstract
This paper proposes a self-filling exhaust-type natural vacuum solar distillation system for seawater desalination, which aligns with China's "green, low-carbon, and efficient" strategy and its "carbon peak and carbon neutrality" goal. The system’s structure and working principle are described, and an experimental apparatus is built to test its water production at different temperatures. The results show that the water production and performance coefficient increase with the water temperature. At a seawater heating mass flow rate of 0.1 kg/s and a temperature of 60℃, the unit cycle water production is 13.77 kg, and the performance factor is 1.80. The paper also analyzes the economic benefits of the system, which aims to improve the desalination speed and rate of seawater, and enhance the wind power generation capacity.
Keywords: Peak carbon dioxide emissions, carbon neutrality, seawater desalination, natural vacuum distillation, economic viability
1. Introduction
Climate change is a global issue that poses a serious threat to the biosphere, as many countries persistently emit CO2 and cause a sharp increase in greenhouse gases. In September 2020, General Secretary Xi Jinping announced China's ambition to peak its carbon dioxide emissions before 2030 and achieve carbon neutrality by 2060 [1]. This is not only a prerequisite for China's sustainable and high-quality development, but also a top priority in building a community of shared future for mankind. Desalination, as an open water supply technology, can augment China’s total freshwater resources and alleviate coastal water scarcity. However, it entails energy consumption during operation and construction, which necessitates the reduction of carbon emissions, the improvement of energy efficiency, and the enhancement of desalination equipment quality to effectively promote industrial standardization. Therefore, research on desalination technology is of paramount importance. The “Outline of the Fourteenth Five-Year Plan for National Economic and Social Development and the Long-Range Objectives Through the Year 2035 of the People's Republic of China” highlights the need to “promote the large-scale development of desalination and ocean energy,” which is one of the greatest challenges China currently faces [2]. In 2021, with the successive release of policy documents on “peaking carbon dioxide emissions” and “achieving carbon neutrality” by the central government and the State Council, changes in China's energy structure, along with improved energy utilization efficiency, will present new opportunities and challenges for China's desalination industry.
Two main methods are adopted by the international community for seawater desalination: thermal methods (multistage flash distillation and low-temperature multiple effect distillation) and membrane methods (reverse osmosis). However, both methods require a certain amount of energy to substitute existing freshwater resources, and the use of conventional energy sources inevitably results in CO2 emissions. Thermal desalination involves both power generation and thermal desalination, while reverse osmosis desalination does not involve phase changes, leading to lower energy consumption and only requiring electricity. The electricity consumption per ton of water is approximately 3-4 kWh. Freshwater resources in coastal, arid, and semi-arid regions in China have become the primary limiting factor for our economic and environmental development. Seawater desalination technology can effectively address this problem in coastal areas, but its development is hindered by high energy consumption and environmental pollution. The use of new energy sources for seawater desalination is an effective way to tackle high energy consumption and pollution. Coastal areas with wind and solar energy resources are ideal locations for seawater desalination. Aznar-Sánchez et al. and others have studied the low-temperature multiple effect distillation seawater desalination process, and found that although this method has very low energy consumption, it still consumes significant energy, and they have pointed out that the water quality from membrane treatment is inferior to that of thermal methods [3]. Guo et al. briefly introduced the situation of seawater desalination worldwide and pointed out that the main challenges currently are excessive energy consumption and significant room for development [4]. Vacuum distillation can achieve seawater desalination, but maintaining the vacuum level requires a large amount of electricity. As the vacuum level increases, both the vacuum rate and power consumption will increase significantly. Choi et al. utilized natural vacuum distillation technology, that is, under the interaction of seawater gravity and atmospheric pressure, to achieve seawater evaporation at room temperature, thereby reducing the use of vacuum pumps [5]. They theoretically studied the desalination process of natural vacuum distillation, and found that evaporation occurs when the seawater pressure is not greater than 4 kPa. They constructed a physical model of this method, and proposed that the technology requires a heat source and the installation of a fan to generate convection, thus improving the system efficiency. Although the power consumption of the system is very low, the power consumption of the auxiliary system is relatively high. Muhammad et al. constructed a mathematical model for solar natural vacuum distillation and based on measurement data from Bahrain, found that this method operates most effectively at a temperature difference of around 20°C [6].
Natural vacuum distillation is a type of vacuum environment generated by natural gravity. It requires high performance of the equipment, using electricity as power without consuming too much electrical energy, achieving significant energy savings. To address the above issues, this paper designs a natural vacuum solar seawater desalination device with water filling and exhaust, which only requires a pipe pump to fill the evaporator with water and discharge the non-condensable gas accumulated in it, thus achieving the purpose of re-evacuation. The system, consisting of an evaporator, a condenser, and several pipelines, is characterized by a simple structure, low cost, low maintenance cost, and long service life. It can effectively solve the problem of domestic water use for residents in isolated and remote areas. The implementation of this project has great scientific value for breaking through China’s green and low-carbon seawater desalination technology, improving energy efficiency, promoting the development of industrial green and low-carbon technology, and advancing the sustainable development of China’s industry.
2. Principle of natural vacuum solar seawater desalination system
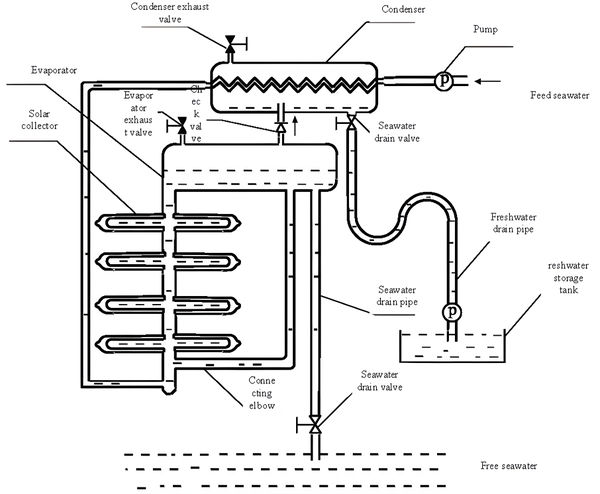
The working principle of the natural vacuum solar seawater desalination system used in this paper is shown in Figure 1.
|
| Figure 1. Schematic diagram of the experimental system |
The system operates as follows: a water supply pump injects seawater into the evaporator at a height exceeding 10m, and then the exhaust valve expels the seawater in the evaporator. This creates a vacuum in the evaporator by the principle of atmospheric pressure supporting a 10m water column. Subsequently, the raw seawater is condensed by the condenser, recovering the latent heat of condensation. It then enters the constant temperature water tank, where it is reheated by the solar collector, and finally reaches the evaporator. To achieve convective heat transfer and enhance heat transfer efficiency, cooling water pipes and hot water seawater inlet pipes are installed on both sides of the evaporator. Under negative pressure, the seawater saturation temperature decreases, and the heated seawater rapidly vaporizes in the evaporator and condenses into fresh water in the upper condenser. The condensed fresh water falls into the pool and flows into the fresh water storage tank through the water pipe by gravity.
Non-condensable gases accumulate in the evaporator during the long-term operation of the device, increasing the pressure inside the evaporator and decreasing the evaporation rate. At this point, water replenishment to the evaporator is required, followed by the next cycle. Specifically, the seawater valve leading to the heat exchanger is closed, and then it is introduced into the evaporator, followed by closing the valve leading to the fresh concentrated seawater, allowing it to continue filling with waste gas, and then releasing the non-condensable gas by approaching a vacuum. If no gas is discharged from the pipeline, the valve of the concentrator hose is removed, and the system returns to a vacuum. The experimental equipment is affected by “twist damage,” which lowers the pressure in the evaporator, thereby reducing the vaporization temperature of seawater in the evaporator, enhancing the evaporation effect of low-temperature seawater, and increasing the fresh water output [7].
The experimental apparatus comprises a stainless steel tank as the evaporator, with a diameter of 350mm and a length of 2000mm, and an outer wall covered by a 20mm rubber insulation layer. The condenser is a stainless steel corrugated tube with a diameter of 25mm and a length of 11m, arranged in a serpentine coil configuration within the evaporator to ensure complete and uniform coverage, with a configuration area of 240mm×1900mm. The water collection area has a V-shaped groove with an included angle of 130 degrees, with a 5-degree tilt to the left and right, facilitating the flow of fresh water within the collection area towards the outlet. A 300W electric heating rod substitutes for traditional solar collectors, with temperature control of the hot water tank achieved using a temperature control box.
The project plans to set up three temperature measurement points at equal distances on the top surface of the evaporator, and to set up temperature measurement points at the inlet of cold seawater, the outlet of concentrated brine, and the outlet to accurately predict the water production performance of the system under different operating conditions. Based on this, the temperature data accuracy of the 32-channel rapid temperature recorder (JLS XMT) is ±0.1℃. A vacuum gauge measures the pressure inside the evaporator, with a measurement range from 0 to 100 kPa and an accuracy of ±1.6%. An electronic scale is installed below the water storage tank, with a measurement accuracy of ±0.5g, to record the water quantity in the system in real time [8]. The temperature of the thermocouple and the consumption of clean water are tested for 1 min and 5 min, respectively, before changing the test formula. If the water quantity in the system is less than 0.1 kg/5min, the end of the system’s operating cycle is determined, and a new round of water injection begins. The schematic diagram is shown in Figure 2.
|
| Figure 2. Temperature measurement point layout |
3. Performance analysis
3.1 Energy analysis of the system
The system’s heat transfer and water production were studied by further analyzing the heat change process and freshwater production process in the system based on the energy conservation equation. In this paper, we assume that the evaporator has good adiabatic characteristics and neglect external heat exchange.
In the evaporator, hot seawater is continuously evaporated, and the energy consumed is the heat of vaporization carried away by the water vapor, as shown specifically in Eq. (1):
|
|
(1) |
where represents the mass flow rate of seawater entering the evaporator at a speed of kg/s, the mass flow rate of concentrated seawater discharged from the evaporator at a speed of kg/s, the specific heat capacity of seawater at constant pressure in units of kj/(kg.k), the water temperature of seawater entering the evaporator in units of ℃, and the water temperature of concentrated seawater discharged from the evaporator in units of ℃.
When seawater is in contact with fresh water and low-temperature condensers in the hot sea, due to the increase in vapor content, the phenomenon of fresh water condensation occurs, and the flow rate of fresh water produced by the device is as shown in Eq. (2):
|
|
(2) |
where represents the latent heat at different condensation temperatures, obtained empirically as Eq. (3):
|
|
(3) |
where represents the condensation temperature of water in degrees Celsius.
When cold seawater flows through the condenser, it enhances the system’s energy efficiency by absorbing the condensation latent heat from the cold water passing through the condenser. The heat gained during this process is represented by Eq. (4):
|
|
(4) |
where represents the temperature of cold seawater flowing out of the condenser in degrees Celsius, and the temperature of cold seawater flowing into the condenser in degrees Celsius.
3.2 Water quality analysis
Seawater desalination can be achieved by two methods: thermal and membrane. The system used in this project is based on the thermal method, which produces freshwater with lower salt content compared to the membrane method. Moreover, it operates at room temperature, unlike conventional thermal techniques. Compared to traditional thermal techniques, the salt molecules have smaller, milder, and higher entropy distributions, resulting in lower salt content in the freshwater [9]. Table 1 shows the water quality of each conventional process, demonstrating that the salt content in the water treated by thermal processes is extremely low. Therefore, the salt content in the factory’s effluent will be reduced to below 5mg/L.
| Traditional seawater desalination technology | Method | Salinity (mg·L-1) |
|---|---|---|
| Low-temperature multiple effect | Thermal method | 5 |
| Multi-stage flash distillation | Thermal method | 5 |
| Vapor compression distillation | Thermal method | 5 |
| Reverse osmosis | Membrane method | 500 |
4. Experimental results and discussion
4.1 Feasibility verification test of the device
Using a wind turbine model with a 150 W electric motor and a simulated vacuum pump, the temperature of unheated fresh water at 25°C was significantly reduced to 25°C after heating to the boiling point, while the temperature of unheated seawater at 25°C was noticeably reduced to 45°C after heating to the boiling point (the temperature indicator of fresh water after heating to the boiling point was unstable, with a large amount of dissolved gas being released).
To investigate the feasibility of the equipment’s working principle, we conducted a series of evaporation experiments using fresh water and seawater at different temperatures. We measured the number of bubbles formed during the evaporation process as an indicator of the amount of dissolved air and the evaporation rate. As depicted in Table 2, we found that fresh water that was heated to boiling and then cooled to 25°C had fewer bubbles than fresh water that was unheated at 25°C, suggesting that heating had removed most of the dissolved air from the water. Dissolved air was more volatile than fresh water and thus evaporated faster. We also observed that seawater at 25°C had fewer bubbles than fresh water at 25°C, indicating that seawater had a lower evaporation rate than fresh water. This could be due to the higher salinity and density of seawater, which reduced its vapour pressure. Furthermore, we compared the temperature of fresh water that was heated to boiling and then cooled to 25°C and 45°C, respectively. We found that the higher temperature increased the evaporation rate, as evidenced by the more rapid decrease in water volume before and after boiling. These results confirmed the validity of the equipment’s working principle.
| Vaporization experiment | Initial observations | Observations after 15 minutes |
|---|---|---|
| Untreated fresh water at 25℃ | Numerous bubbles with diameters of 3.5cm and 1cm | Decrease in the initial bubble quantity |
| Fresh water heated to boiling and then cooled to 25℃ | Numerous bubbles with diameters of 3.5cm and 1cm | Decrease in the initial bubble quantity |
| Untreated seawater at 25℃ | Few bubbles with diameters of 3.5cm and 1cm | Decrease in the initial bubble quantity |
| Seawater heated to boiling and then cooled to 25℃ | Vigorous boiling | Almost no change in the initial bubble quantity |
4.2 Desalination experiment simulation
Seawater evaporation under different wind speeds at room temperature (25°C) was investigated using a cylindrical evaporator. The wind speeds were varied incrementally from the previous limit, and the experimental results are presented in Figure 3. As shown in Figure 3, the evaporation rate of seawater increased with the wind speed, matching the pumping rate of the vacuum pump, which resulted in a decrease and stabilization of the air pressure at a certain value. The time required to reach the maximum pressure for each wind speed was shorter than that for the original pressure, as the seawater evaporated faster at lower pressure, replenishing the air pressure more rapidly. The gas pressure reached a steady state in a shorter time span as the evaporation rate increased.
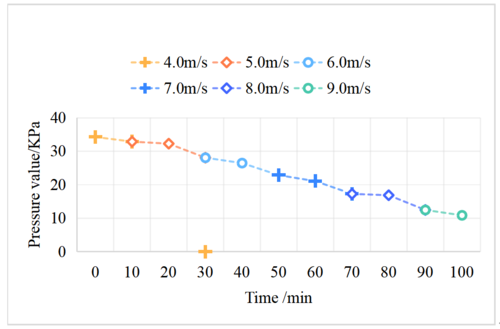
|
| Figure 3. Relationship between air pressure and wind speed |
The boiling point of seawater varies depending on its composition and concentration, which differ across regions, and there is no standard reference for it. Therefore, we used the boiling point of fresh water at the corresponding limit pressure as a reference to estimate the critical pressure values obtained at different velocities, as shown in Figure 4. The critical pressure decreased and stabilized as the velocity increased. The boiling point of fresh water also decreased gradually, indicating that the same trend would apply to seawater.

|
| Figure 4. Boiling point of fresh water at the corresponding wind speed and corresponding air pressure limit pressure |
The experimental phenomena and data presented above lead to the following conclusions: the critical pressure is inversely proportional to the wind speed and the evaporation rate is directly proportional to it; both fresh water and seawater exhibit abundant bubbling in the initial stage of evaporation due to the release of gases during the process; the evaporation rate of seawater can be significantly enhanced by heating; the evaporation rate also depends on the type of liquid, resulting in different maximum pressures that can be achieved by various liquids in the cylindrical evaporation chamber under the same flow rate; a rotary vane vacuum pump was chosen as the equipment model, which has a relatively small pumping chamber, requiring a high rotational speed to increase the pumping speed. In the experiment, the wind power was sufficient, exceeding 150 W, but the wind turbine had a corresponding speed for each wind speed. However, the rotational speed of the wind turbine was still insufficient for the vacuum pump, and a lot of wind energy was wasted, making it difficult to lower the limiting pressure and to evaporate seawater at room temperature. Therefore, the suggested improvement for this model is to select a larger vacuum pump or to install larger gears on the original vacuum pump to better choose the type and size of the blades, and to design the cylindrical steam chamber size rationally.
4.3 The system’s water production rate under continuous cycling conditions
The above experiment used experimental seawater as the raw material and applied a constant temperature control to the hot box using a temperature control box. The preparation process involved inflating and draining the evaporator for about 20 minutes to establish a vacuum inside. After the discharge was completed, the evaporator could generate an internal absolute pressure of about 8 kPa. Figure 5 shows the temperature change of the top outer wall of the evaporator with the temperature, with seawater heated to 50°C and a mass flow rate of 0.1 kg/s. The experimental results indicate that the temperature of the outer wall of the evaporator first increases sharply, and then decreases sharply during the second filling process, exhibiting a complete cycle process [10]. When the heated seawater is injected into the evaporator, it undergoes instant boiling when it reaches the saturation temperature corresponding to its internal pressure, with some of the steam being condensed into fresh water in the condenser, while the remaining hot steam and non-condensing gas accumulate at the top of the evaporator under the influence of buoyancy, thereby increasing the temperature and pressure inside the evaporator. It can be observed that the temperature of the evaporator wall () is lower near the cold water inlet, indicating higher cooling efficiency, as cold water differs from hot water.
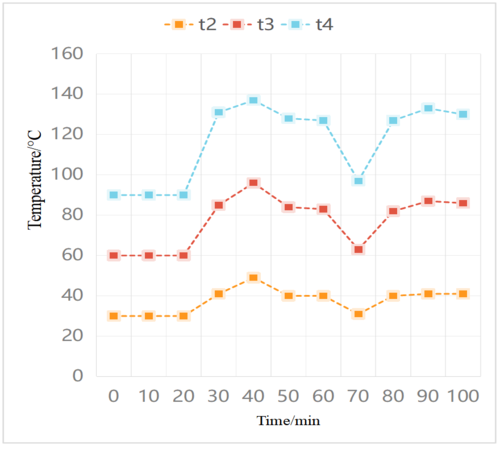
|
| Figure 5. Variation of the temperature at the top of the evaporator |
Figure 6 illustrates the relationship between the outlet temperature of each pipeline and time. It can be seen from Figure 6 that the outlet temperature of concentrated seawater increases with time, indicating that the evaporation rate of high-temperature seawater continues to decrease. Consequently, at the end of the cycle, has risen to 47℃. The outlet temperature of cold seawater sharply rises and then gradually decreases because when the hot water enters the low-pressure evaporator, it evaporates rapidly. The vapor is significant, and when it comes into contact with the condenser, latent heat is rapidly released, condensing into fresh water, causing the temperature to rise rapidly. The smaller the evaporation coefficient, the less heat transfer occurs between the steam and the condensing tube, resulting in a smaller . Additionally, since the temperature of the fresh water from condensation is mainly influenced by the temperature of the condensing tube, the trend of the water outlet temperature is similar to the trend of but with a much smaller magnitude of change.

|
| Figure 6. Variation of outlet temperatures for each pipeline |
The pressure and instantaneous water production of the two cycles inside the evaporator were theoretically calculated and the results were consistent with the experimental results. Based on this, a new design scheme was proposed according to the experimental results, adopting a new design. The pressure in the distillation tower increased rapidly at the beginning of heating the water, and the yield was also high, producing 1.05 kg of fresh water in 5 minutes. However, as the pressure increased, the water production rate gradually decreased. This was mainly attributed to the fact that in the distillation device, the overflow speed of steam and non-condensable gas was very fast when the steam was at very low pressure. When the steam was condensed into fresh water, the accumulation of steam in the distillation device increased. During this process, the non-condensable gas hindered the condensation heat transfer of water vapor in the condensation tube, causing a large amount of water vapor to accumulate in the evaporator, resulting in an increase in pressure and a decrease in water production. As shown in Figure 4, at a temperature of 50°C, when the hot seawater entered the equipment, the low temperature was severely affected by the non-condensable gas, so after excluding the time for filling and draining water, each cycle could only operate for 35 minutes, which was not conducive to the equipment operation.
4.4 Comparison of water production performance at different hot sea water temperatures
Different temperatures of warm sea water will have a significant impact on the system’s operational capacity. Under the condition of constant water supply of 0.1 kg/s, the relationship between the internal pressure of the evaporator and the high-temperature sea water is shown in Figure 7.
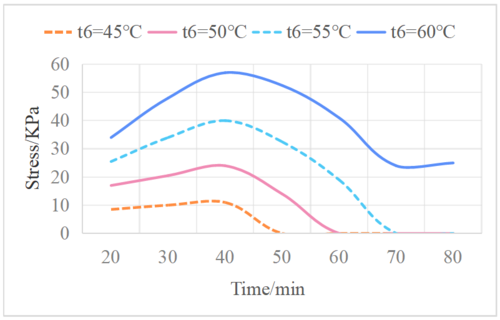
|
| Figure 7. Pressure at different hot sea water temperatures |
Figure 7 illustrates that the working cycle time of the system and the degree of pressure increase both decrease as the temperature of the hot sea water decreases. This is because the reaction with non-condensing gas is faster at lower water temperatures. Due to the lower temperature, less sea water evaporates, and although the emission of non-condensing gas is also small, its proportion of the vapor is large, thus shortening the operation time [11]. Figure 8 shows the cumulative water production of the system corresponding to different water temperatures. The cumulative water production of the system increases with the increase of water temperature. Under the condition of water temperature of 60°C, the system has a working cycle of 75 minutes, and a cumulative water production of 13.77 kg, which means it produces 10.98 kg of fresh water per hour.
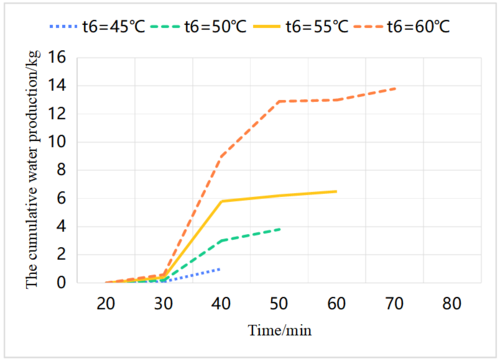
|
| Figure 8. Cumulative water production at different hot seawater temperatures |
The performance coefficient (GOR) expresses the performance evaluation index of the system. According to the energy cost/production ratio, the equipment efficiency coefficient is the ratio of the latent heat required for freshwater production, which can be calculated using Eq. (5):
|
|
(5) |
According to Eq. (5), the performance coefficient of the system at different water temperatures is shown in Table 3. From Table 3, it can be seen that the heat transfer coefficient increases with the rise of water temperature, and the heat transfer coefficient can be as high as 1.80. This is because as the water temperature increases, the heat transfer between steam and condenser increases, thereby improving the condensation efficiency. Improving the fresh water efficiency can allow cold water to absorb more evaporative potential, thereby improving energy efficiency.
| Temperature of hot spring water (°C) | Amount of water produced per cycle (kg) | Cycle time /min | GOR |
|---|---|---|---|
| 45 | 1.03 | 40 | 1.62 |
| 50 | 3.53 | 55 | 1.65 |
| 55 | 7.12 | 65 | 1.72 |
| 60 | 13.77 | 75 | 1.80 |
5. Economic analysis of the system
As a solar-powered seawater desalination device, its economic performance directly affects whether it can be commercialized and widely promoted, so the study of its economic performance is particularly important. Here, as represented by Eqs. (6) to (7), total cost () consists of fixed costs () and variable costs (). Fixed costs are the purchase and installation costs of each device, while variable costs are maintenance costs caused by system losses:
|
|
(6) |
|
|
(7) |
where represents the annual loss factor of the system, including the losses of various components such as pumps, evaporators, etc., and represents the effective lifespan of the system, denoted as ''. By using Eq. (8), calculate the number of flat solar panels required for the system:
|
|
(8) |
where represents the area of each panel, m2, is the number of different types of flat plate collectors, represents the average intensity of sunlight, , and represents the average efficiency of solar cells, which is 0.5% per day.
If the required system can produce 10.98kg of fresh water per hour, it means that under the conditions of a flow rate of 0.1kg/s, a hot seawater temperature of 60°C, and an average daily solar radiation intensity of 850w/m2, the number of solar collectors required can be calculated as 6 using formula (8). The construction costs of each system component are listed in Table 4, and the total fixed costs calculated in the following manner are yuan/ton. Due to the fewer system components and the scarcity of system elements and parts, it is assumed that the annual value is 10% of , i.e., S=0.1.
| No. | Component name | Fixed cost (yuan/ton) |
|---|---|---|
| 1 | Evaporator | 900 |
| 2 | Condenser | 150 |
| 3 | Freshwater collection tank | 100 |
| 4 | Solar collector | 6×350 |
| 5 | Circulating water pump | 120 |
| 6 | Valves and pipelines | 130 |
| 7 | Installation cost | 500 |
| 8 | Total fixed cost | 4000 |
Conservatively estimating the service life of the system to be 18 years, with yuan/ton, the city experiences over 300 clear days per year, allowing for continuous operation for 8 hours a day. The daily production of fresh water is kg, resulting in a total fresh water production of 474.34 tons over 18 years. As a result, the average cost of producing one cubic meter of water is 22.5 yuan/ton, which is lower than the conventional solar distillation system (33.6 yuan/ton). Although this cost is relatively high compared to tap water in the city, it is still acceptable when compared to the cost of pure water at 400 yuan/ton.
6. Conclusion
This paper presents a solar-powered seawater desalination device with a water-filled exhaust-type natural vacuum distillation system, which can desalinate seawater using solar energy. The seawater enters the device and passes through a condenser first, then enters the solar collector, where the water temperature is raised. The high-temperature seawater boils in the vacuum evaporator, accelerating the evaporation rate. The generated water vapor flows through pipelines and one-way valves into the condenser, where it condenses into fresh water and heats the low-temperature seawater inside the condensation tube. The desalinated water then flows into a freshwater storage tank through pipes. Part of the evaporated seawater is discharged into the sea, while the rest returns to the solar collector through natural convection. The device also uses the water-filling method to discharge non-condensable gases from the device, thereby achieving vacuum restoration. Experimental research has been conducted on this basis, leading to the following conclusions:
(1) By using the natural negative pressure principle, the internal absolute pressure of the evaporator can reach 8 kPa. During the system operation, the accumulation of non-condensable gases causes an increase in pressure inside the evaporator. In this situation, a pump can be used to separately inject seawater and fresh water into the evaporator and condenser, and an exhaust valve can be used to discharge non-condensable gases from the evaporator and condenser. Subsequently, the exhaust valve is closed and the drain valve is opened, restoring natural vacuum to the evaporator and condenser, allowing the entire system to continue operating, although the water production rate decreases continuously.
(2) Under the condition of 60°C, the cycle time is 75 minutes, with a water production of 13.77 kg per cycle and a corresponding GOR of 1.80. This is because the heat transfer between the steam and condenser increases as the water temperature increases, thereby improving the condensation efficiency and increasing the fresh water production. This allows the cold water to absorb more evaporative potential, thus improving the energy efficiency.
(3) An economic analysis of the natural vacuum seawater desalination system revealed a fixed cost of 4000 yuan, and the average cost of producing fresh water over the entire lifecycle is 22.5 yuan per ton. It is evident that the entire device consists of simple structures, with the advantages of low cost and stable operation. Additionally, the entire system operates at medium to low temperatures, resulting in low corrosion of seawater and a long system lifespan. It is suitable for large-scale applications in factories as well as for individual use in remote areas, islands, and similar locations, presenting broad market prospects.
Acknowledgements
This study was supported by Shaanxi Provincial Social Science Foundation (No:2018D13) and The Shaanxi Soft Science Research Foundation (No:2020KRM152)
References
[1] Xi J.P. Speech at the general debate of the 75th session of the United Nations General Assembly. Gov. Gaz. People’s Repub. China, 28:1-3, 2020.
[2] Shi H. Research on carbon footprint accounting method for petroleum and chemical enterprise products. Pet. Petrochem. Mater. Proc., 16:161–162, 2021.
[3] Aznar-Sánchez A., Belmonte-Urea L.J., Velasco-Muñoz J.F., Valera D.L. Farmers’ profiles and behaviours toward desalinated seawater for irrigation: Insights from South-east Spain. J. Clean. Prod., 296, 126568, 2021.
[4] Guo C., Zhao J., Zhang T. Development of a modified composite structure from polyurethane sponge for desalinating seawater polluted by oil. Desalination, 524, 115471, 2022.
[5] Choi W.Y., Aravena C., Park J., Kang D., Yoo Y. Performance prediction and evaluation of CO2 utilization with conjoined electrolysis and carbonation using desalinated rejected seawater brine. Desalination, 509, 115068, 2021.
[6] Muhammad Q., Mohamed B., Noor A. Reverse osmosis desalination: A state-of-the-art review. Desalination, 459:59–104, 2019.
[7] Wu X., Liu W., Gao H., Alfaro D., Sun S.R., Lei R.R., Jia T.Q., Zheng M.H. Coordinated effects of air pollution control devices on PAH emissions in coal-fired power plants and industrial boilers. Sci. Total Environ., 756, 144063, 2021.
[8] Wu S., Su L., Ge Y. Assessment of carbon footprint and emission reduction strategies for seawater desalination lifecycle. Ecol. Econ., 261:24–26, 2012.
[9] Li Z., Zhang D., Pan L. Low-carbon energy transition path and suggestions for China under the “dual carbon” target. J. Power Eng., 41:905–909, 2021.
[10] Zuo L., Zhou T., Li C. Techno-economic analysis of wind-assisted solar chimney power plants with combined seawater desalination and waste heat utilization. Renew. Energy, 40:1459–1464, 2022.
[11] Liu S., Zhang F., Wang J., Chen A., Li L. Research on carbon reduction paths and strategies for seawater desalination in China under the “dual carbon” target. Environ. Sci. Manag., 47:15–19, 2022.Document information
Published on 15/05/24
Accepted on 02/05/24
Submitted on 20/04/24
Volume 40, Issue 2, 2024
DOI: 10.23967/j.rimni.2024.05.002
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?