(Created page with " ==Highlights== * Fibrotic disorders increase in incidence/prevalence with aging. * Idiopathic pulmonary fibrosis (IPF), an age-related lung disease, is associated with oxida...") |
m (Scipediacontent moved page Draft Content 882063817 to Kurundkar Thannickal 2016a) |
(No difference)
| |
Latest revision as of 15:52, 20 October 2016
Highlights
- Fibrotic disorders increase in incidence/prevalence with aging.
- Idiopathic pulmonary fibrosis (IPF), an age-related lung disease, is associated with oxidative stress.
- The mechanistic links between aging and IPF are not well understood.
- Age-associated redox imbalance contributes persistent fibrosis in IPF.
Abstract
Redox signaling and oxidative stress are associated with tissue fibrosis and aging. Aging is recognized as a major risk factor for fibrotic diseases involving multiple organ systems, including that of the lung. A number of oxidant generating enzymes are upregulated while antioxidant defenses are deficient with aging and cellular senescence, leading to redox imbalance and oxidative stress. However, the precise mechanisms by which redox signaling and oxidative stress contribute to the pathogenesis of lung fibrosis are not well understood. Tissue repair is a highly regulated process that involves the interactions of several cell types, including epithelial cells, fibroblasts and inflammatory cells. Fibrosis may develop when these interactions are dysregulated with the acquisition of pro-fibrotic cellular phenotypes. In this review, we explore the roles of redox mechanisms that promote and perpetuate fibrosis in the context of cellular senescence and aging.
Graphical Abstract
Keywords
Oxidative stress ; Senescence ; Myofibroblasts ; Inflammation ; Epithelial cells
1. Introduction
Tissue repair is an evolutionally conserved and tightly regulated process that aims to reinstate tissue integrity and functional capacity of the affected organ. When this process is uncontrolled, fibrotic repair with attendant loss of organ function may occur. Fibrosis is excessive accumulation of extracellular matrix (ECM), due to sustained mesenchymal activation and impaired epithelial regeneration with varying degrees of inflammation. Fibrosis can affect diverse organs such as lung, liver, kidney and skin [1] . Incidence and prevalence of pathological fibrosis increase with advancing age. Aging is an independent risk factor for fibrotic disorders [2] , [3] , [4] , [5] , [6] , [7] and [8] . However, the mechanistic links between aging and fibrosis are not well understood [9] .
Both aging and fibrosis are associated with redox imbalance and oxidative stress [10] and [11] . Redox imbalance/oxidative stress occur when there is increased production and inefficient scavenging of reactive oxygen species (ROS) [12] . There is convincing evidence of increased oxidative stress in fibrotic disorders [13] and [14] , although precise roles of oxidative stress in the pathogenesis of fibrosis have not been well-defined [15] , [16] and [17] . Similarly, while there is debate about the casual role of ROS in aging, there is compelling evidence that aging is associated with oxidative stress [18] . Can redox imbalance be the mechanistic link between aging and fibrosis? In this review, we evaluate and discuss the currently available evidence linking oxidative stress and redox mechanisms to age-related fibrosis.
2. Fibrosis is a disease of aging
Aging is a gradual decline in the functional capacity of an organism predisposing to death [19] . IPF is a disease of aging [9] and [20] , with increasing incidence and prevalence in human subjects over the age of 50 years [2] , [4] , [21] and [22] . Additionally, older individuals with IPF have significantly diminished survival [23] and [24] . Individuals with progeria syndromes are also at higher risk of fibrotic disorders [25] and [26] . Recent studies indicate that shortened telomere length is a risk factor for IPF [27] , and IPF is the most common disorder in telomere dysfunction syndromes [28] . The risk of liver fibrosis with chronic viral infections and alcohol exposure also increase with aging [29] . Increasing age is associated with renal glomerulosclerosis and cardiac fibrosis [5] and [7] . These studies suggest that fibrosis involving diverse organ systems may represent a degenerative disorder of advancing age. The reasons why older individuals are prone to fibrosis are still not clear. It is possible that the altered redox balance with aging skew the cascade of normal tissue repair responses towards fibrosis.
3. Redox mechanisms in fibrosis
Molecular oxygen is used by aerobic organisms to harness energy from oxidation of energy-rich compounds as a part of normal metabolism. During this process of oxidative phosphorylation, highly reactive and partially reduced ROS that include hydrogen peroxide (H2 O2 ), superoxide anion (O2−∙ ), and hydroxyl radical (OH∙ ) may be generated [12] . These ROS can damage cellular biomolecules such as lipids, protein, and DNA. Excessive exposure to ROS induces oxidative stress and results in apoptosis, senescence and/or genetic mutations [30] . To combat increases in ROS, antioxidant systems [glutathione (GSH), catalase, superoxide dismutase (SOD2), nuclear factor erythroid-2 (Nrf-2)] have evolved [31] . Organisms have also developed purposeful ROS-producing enzymes (e.g., NADPH oxidases and myeloperoxidases) that participate in host defense functions and in cellular signaling [12] . The apparent contradictory roles of ROS as essential regulators of cellular function vs. cytotoxic mediators are, at least in part, attributable to their concentrations and cellular compartmentalization [12] . Additionally, these biomolecules can function in a contextual and antagonistically pleiotropic manner in aging [32] and [33] .
Tissue repair is an orchestrated process that involves recruitment, proliferation, differentiation, deactivation, and apoptosis of different cell types [34] . Cellular senescence is one of the hallmarks of aging [19] and can profoundly influence the tissue repair process [35] . Fibroblast senescence may restrict the proliferation of fibroblasts and promote resolution of fibrosis [35] and [36] . In contrast, senescence of epithelial cells can result in diminished capacity of the regeneration [37] and [38] , and persistent senescence of fibroblasts can result in impaired clearance due its decreased apoptosis susceptibility in age-related lung fibrosis [39] and [40] . Cellular senescence has been associated with increased oxidative stress [41] . ROS has been shown to regulate the cellular phenotypes and fates, including differentiation [42] and [43] , apoptosis [44] , migration [45] and [46] , and invasion [47] during tissue repair. There is growing evidence of a pathological role of redox imbalance and oxidative stress in fibrotic repair [39] and [48] . It is plausible that redox imbalance associated with aging alters redox signaling during tissue repair, thus, predisposing to non-resolving fibrosis.
4. Cell-specific phenotypes/fates in fibrotic repair
Dysregulated tissue repair may ensure as a result of intrinsic changes in specific cell population or due to dysregulated cell-cell communication. Cellular phenotypes/fates that are consistently observed in fibrotic repair include: [1] impaired epithelial regeneration with enhanced apoptosis susceptibility; [2] accumulation of mesenchymal cells with an apoptosis resistant phenotype; and [3] chronic low-grade inflammation and features of immunosenescence. Here, we will discuss how redox dysregulation contributes to these cellular phenotypes and fates associated with age-related fibrosis (Fig. 1 ).
|
|
|
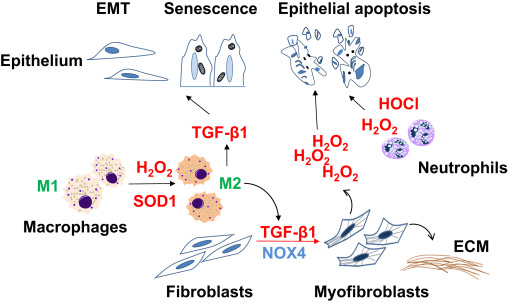
Fig. 1. Cell-specific phenotypes and fates in fibrotic repair Impaired reepithelialization in fibrotic repair may occur as a result of apoptosis, epithelial-to-mesenchymal transition (EMT), and/or senescence. Transforming growth factor-β1 (TGF-β1), a critical pro-fibrotic cytokine, mediates differentiation of fibroblasts to myofibroblasts, the primary cells that secrete and remodel the extracellular matrix (ECM). Myofibroblasts may induce epithelial cell death through NADPH oxidase 4 (NOX4)-dependent H2 O2 production. Reactive oxygen species in form of hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) generated by neutrophils and macrophages may contribute to epithelial injury. Superoxide dismutase-1 (SOD1)-catalyzed H2 O2 production mediates transition of M1 macrophages to M2 macrophages, which secrete TGF-β1 and promote pro-fibrotic cellular phenotypes through paracrine effects. |
4.1. Epithelial cells
The alveolar epithelial lining of the lung is covered by type I pneumocytes (~95% of surface area) and type II pneumocytes (~5% of surface lining). Alveolar epithelial cells (AECs) are the primary site of injury and thought to trigger/initiate the fibrotic response [49] . While type II AECs proliferate and differentiate to type I AECs in response to injury, AECs in fibrotic repair appear prone to undergo apoptosis [50] and [51] and/or senescence [52] and [53] . AECs secrete several growth factors/cytokines, such TGF-β1 [54] , platelet-derived growth factor (PDGF) [55] , endothelin-1 [56] , angiotensin II [57] , and connective tissue growth factor (CTGF) [58] ; these cytokines can directly and indirectly mediate pro-fibrotic activation of mesenchymal cells. Apoptosis of AECs has been shown to initiate fibrosis [49] , [59] and [60] , and secreted factor(s) from senescent epithelial cells can induce myofibroblast differentiation in-vitro [53] . Epithelial cells may potentially undergo phenotypic transitions to mesenchymal-like cells and contribute to fibrogenesis, most likely through secreted mediators [61] , [62] and [63] . Thus, AECs are not just targets but active participants in the fibrogenic process.
Mitochondrial ROS has been implicated in pathophysiological process of many age-related degenerative diseases [64] . The epithelial cell damage seen in IPF is associated with increased mitochondrial ROS [65] . Smoking, a known risk factor for IPF, can induce epithelial cell senescence in lung by increasing mitochondrial ROS [66] . Mitochondrial ROS has also been shown to be critical for hypoxia-induced alveolar epithelial-mesenchymal transition [67] . TGF-β1, a key pro-fibrotic cytokine [68] and [69] , has been shown to induce apoptosis as well as epithelial mesenchymal transition (EMT) in lung epithelial cells [62] , [70] and [71] . ROS plays an important role in TGF-β1-induced EMT and H2 O2 mimics effects of TGF-β1 in epithelial cells [72] . TGF-β1 has been shown to induce senescence in lung epithelial cells by upregulation of mitochondrial ROS [73] .
Mitochondria show signatures of oxidative damage and large number of mutations with advancing age [74] and [75] . AECs from IPF lungs show accumulation of dysfunctional mitochondria [76] . Persistent mtDNA damage can activate intrinsic apoptotic death pathway due to the collapse in mitochondrial membrane potential [77] and [78] . AECs in IPF lung show evidence of mitochondrial dysfunction characterized by low expression of PTEN-induced putative kinase 1(PINK1) protein [76] , a key protective mechanism by promoting mitophagy in response to oxidative stress [79] and [80] . Expression of PINK1 is significantly diminished in the aging murine lung and may contribute to mitochondrial dysfunction; type II AECs isolated from PINK-deficient mice demonstrate mitochondrial dysfunction and are more susceptible to apoptosis [76] . Another mitochondrial protein, ROS modulator 1 (Romo1) which is highly expressed in AECs of IPF lung, mediates apoptosis in response to oxidative stress [81] . Romo1 expression is increased with replicative senescence [82] . These studies suggest that aging could increase the susceptibility of AECs to oxidative stress, at least in part, due to deficiency of PINK1 and increased expression of Romo1 and, thus, promote fibrotic repair following injury.
AECs express high levels of caveolin-1, a primary structural protein of caveolae in plasma membranes, which regulates signal transduction by its scaffolding properties [83] and [84] . In several cell types, caveolin-1 expression increases with aging [85] and [86] . Premature senescence is induced by oxidative stress, and this effect is at least in part mediated by caveolin-1 sequestration of sirtuin-1 and inhibition of its activity [87] . Loss of caveolin-1 limits premature stress-induced senescence in epithelial cells and protects from bleomycin-induced fibrosis [88] ; whether increased levels of caveolin-1 in senescent AECs contributes to age-related lung fibrosis remains to be determined.
Duox1 and Duox2, members of the family of NADPH oxidase (NOX) enzymes, are expressed in airway epithelia and contribute to H2 O2 release is response to different stimuli [89] and [90] ; however, their role(s) in fibrosis is not known. Another NOX family member, NOX4 has been implicated in injury-induced epithelial cell death and bleomycin-induced fibrosis [91] . Role of NADPH oxidases in epithelial senescence or aging has not been elucidated. Further studies are required to determine the precise roles of NOX enzymes in epithelial senescence and fibrosis. Nuclear factor, erythroid-derived 2, like 2 (Nrf2) is a redox-sensitive transcription factor that is involved in the activation of antioxidant response elements (ARE) and induction of antioxidant/defense genes including glutathione-S-transferase (GST), NADP(H): quinone oxidoreductase 1 (NQO1), thioredoxin, and glutathione peroxidase (GPx). AECs isolated from Nrf2-deficient mice are prone to oxidant-induced cell death and impaired proliferation [92] ; mice with deficient Nrf2 have increased fibrosis in response to bleomycin lung injury [93] . Nrf2 activity declines with aging [94] , [95] and [96] . Impaired Nrf-2 activation in lung fibroblasts has been shown to contribute to persistent fibrosis in aged mice [39] ; however, the precise role of Nrf2 signaling in AECs senescence or age-related fibrosis has not been explored.
Glutathione (GSH), an antioxidant tripeptide, is present in the epithelial lining fluid of the normal lower respiratory tract [97] . Epithelial lung fluid of IPF patients contains lower levels of GSH, suggesting increased susceptibility to oxidant-induced epithelial damage [97] and [98] .
TGF-β1 suppresses gene expression of glutamate cysteine ligase (GCL), a key enzyme in the biosynthesis of GSH, in alveolar epithelial cells [99] and [100] . Overexpression of TGF-β1 by intranasal administration of an adenovirus expressing constitutively active TGF-β1 induces lung fibrosis, and is associated with downregulation of GCL gene expression, decreased GSH levels in bronchoalveolar lavage, and increased oxidative stress [101] . The adaptive GSH antioxidant response to cigarette smoking is significantly decreased in aged mice as compared to young mice [102] . Another antioxidant defense pathway, the thioredoxin (Trx) system, is activated during oxidative stress [103] ; although it is strongly expressed in metaplastic alveolar epithelium, its expression is reduced in the fibrotic lesions of IPF subjects [104] . These studies suggest that aging-induced deficiencies in the antioxidant system may predispose lung epithelial cells to injury and fibrosis. In contrast, manganese superoxide dismutase (Mn-SOD), a mitochondrial antioxidant enzyme, is highly expressed in type II pneumocytes and alveolar macrophages in IPF [105] . This response likely reflects ineffective efforts to curb oxidative stress associated with this age-associated lung disease. Thus, ROS generation from mitochondria and NOX enzymes can influence pro-fibrotic AEC fates such as apoptosis, senescence and EMT (Fig. 2 ).
|
|
|
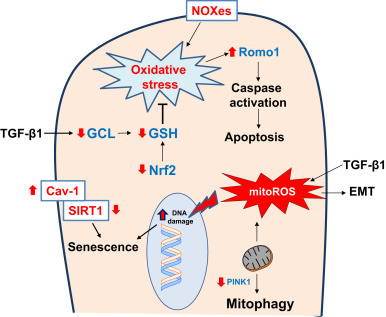
Fig. 2. Redox alterations in epithelial cells in age-related fibrosis Reactive oxygen species (ROS) generated from NADPH oxidase enzymes (NOXes) and mitochondria, along with deficient antioxidant pathways (Nuclear factor erythroid-2 related factor 2, Nrf2; and glutathione, GSH), results in oxidative stress. Transforming growth factor-β1 (TGF-β) suppresses gene expression of glutamate cysteine ligase (GCL), a key enzyme in the biosynthesis of GSH and thus, increases susceptibility to oxidative damage. Oxidative stress leads to epithelial cell death that has been attributed to a deficiency in phosphatase and tensin homolog (PTEN)-induced putative kinase-1 (PINK1) and increased expression of reactive oxygen species modulator-1 (Romo1) in mitochondria. Oxidative stress may also induce epithelial senescence through caveolin-1 (Cav-1)-mediated inhibition of sirtuin-1 (SIRT1) activity. TGF-β1 induced mitochondrial ROS (mitoROS) has been reported to induce epithelial-to-mesenchymal transition (EMT). These redox alterations in epithelial cells promote pro-fibrotic phenotypes such as epithelial apoptosis, EMT and senescence. Red arrows indicate direction of change in age-related fibrosis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
4.2. Fibroblasts
Fibroblasts are key effector cells in fibrotic remodeling and repair [106] . The epithelial injury is followed recruitment and proliferation of fibroblasts and their differentiation to myofibroblasts [107] . Myofibroblast are activated fibroblasts with increased contractile function, increased ECM production, higher oxidant production and decreased apoptosis susceptibility [106] . Several sources of (myo)fibroblast have been proposed and these include fibroblasts, epithelial cells, endothelial cells, fibrocytes and pericytes [107] . Fibroblastic foci, an aggregation of myofibroblasts, are a key feature of IPF and the extent of the fibroblastic foci strongly correlates with impaired pulmonary function and disease progression [108] , [109] and [110] . While the transient appearance of myofibroblasts are essential in normal wound healing, the persistence of myofibroblasts in the injured tissue is a consistent finding in chronic fibrotic disorders [111] . The senescence of fibroblasts confers an apoptosis resistant phenotype and contributes to persistent fibrosis in aged mice [39] . Similarly, IPF myofibroblasts ex-vivo demonstrate a senescent phenotype compared to normal lung fibroblasts [112] , and exhibit apoptosis resistant phenotype in-vitro [39] and [51] . In IPF lung, (myo)fibroblasts in fibroblastic foci show low expression of proliferation marker (Ki67) and higher expression of the senescent marker, p16 [39] . Senescent fibroblasts isolated from old mice are apoptosis resistant in-vitro and accumulate following bleomycin injury in-vivo [39] and [40] . These studies suggest that IPF may result from the accumulation and activation of senescent, apoptosis-resistant myofibroblasts.
Mitochondria are the major source of ROS production in eukaryotic cells. Mitochondrial ROS production increases with replicative senescence and with aging [113] . TGF-β1 increases mitochondrial ROS which has been shown to induce the expression of pro-fibrotic genes during myofibroblast differentiation [114] . The matricellular protein CCN1, which is known to induce mitochondrial ROS [115] , has been shown to augment TGF-β1 signaling and contribute to fibrogenic responses to lung injury [116] . Interestingly, CCN1 expression increases in replicative senescent fibroblasts [117] , and with aging [118] and [119] . The in-vivo roles of mitochondrial ROS in development and/or progression of fibrosis require further study.
Peroxisomes are small single membrane, self-replicating organelles that contain enzymes for β-oxidation of fatty acids and catalase for H2 O2 detoxification [120] . Compromised peroxisomal function can lead to increased cellular ROS and induce pro-fibrotic responses by activation of TGF-β1 signaling [121] . Senescent lung fibroblasts are associated with decreased peroxisomal catalase activity and increased ROS production [122] . Hypocatalasemic, individuals with reduced cellular catalase levels, experience premature onset of age-associated diseases such as type-2 diabetes and macular degeneration [123] and [124] . It remains to be determined whether compromised peroxisomal function with aging results in reduced catalase levels, oxidative stress and susceptibility to fibrosis.
Caveolin-1 expression is decreased in myofibroblasts in IPF [125] , and in response to TGF-β1 [126] . Cav-1 negatively regulates NOX-derived ROS by altering enzymatic activity, post-transcriptionally by direct binding of NOX2 and NOX5 and transcriptionally by inhibiting NF-kB-dependent NOX2 and NOX4 expression [127] , suggesting one mechanism for increased expression of NOXes in IPF. However, caveolin-1 expression increases with replicative senescence in fibroblasts [86] in association with increased expression of NOX4 [128] .
NOX4 mediates myofibroblast differentiation through the generation of H2 O2 , and this redox signaling pathway is required for fibrogenic responses to injury [43] . NOX4 expression is increased in different tissues with aging [129] and [130] , and with cellular senescence in association with epigenetic changes [128] . NOX4 expression is increased in IPF fibroblasts and is required for constitutive expression of their pro-fibrotic phenotype [131] . NOX4 knockdown or pharmacological inhibition of its activity in aged mice restores the capacity for fibrosis resolution [39] .
Nrf2 is a key transcriptional activator of antioxidant genes and maintains redox balance [132] and [133] . Nrf2 has been shown to protect against pulmonary fibrosis in murine models [93] and [134] . IPF myofibroblasts express low levels of Nrf2 in comparison to control fibroblasts, and sulforaphane (an Nrf2 activator) induces dedifferentiation of myofibroblasts isolated from IPF patients [135] . Nrf2 may induce paradoxical amplification of ROS through the stimulation of transcription Kruppel-like factor 9 (Klf9) which regulates a number of redox-modulating enzymes [209] . Apoptosis of myofibroblasts is critical for resolution of fibrosis [51] , and senescent fibroblasts are resistant to apoptosis [39] , [40] and [86] . Nrf2 response to oxidative stress is impaired in senescent fibroblasts in comparison to non-senescent fibroblasts [39] . The ability to induce Nrf2 in fibroblasts isolated from young mice was found to be critical for apoptosis susceptibility [39] . Altered redox homeostasis resulting from elevated expression of NOX4 and a diminished ability to induce Nrf2 antioxidant response leads to non-resolving fibrosis in aged mice [39] . Further studies are required to determine whether Nrf2 activation is a beneficial therapeutic strategy for age-related fibrosis.
Altered interactions between epithelial cells and mesenchymal cells can promote pro-fibrotic environment [53] , [136] and [137] . There is increased epithelial cell death in areas adjacent to myofibroblastic foci in the lungs of IPF patients [138] . Myofibroblasts can induce epithelial cells apoptosis by paracrine H2 O2 signaling in an ex-vivo co-culture system of lung epithelial cells and IPF myofibroblasts [139] . Senescent fibroblasts generate higher levels of extracellular H2 O2 mediated, at least in part, by NOX4 [39] and [128] . It is possible that with aging, senescent fibroblasts produce enhanced H2 O2 that contributes to the oxidative microenvironment in fibrotic disorders. The sources of ROS in (myo)fibroblasts may include mitochondria, NOX enzymes and peroxisomes (Fig. 3 ).
|
|
|
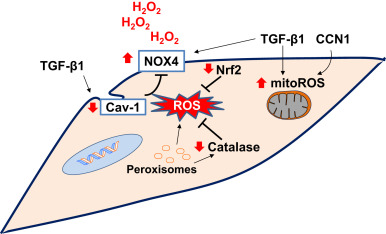
Fig. 3. Redox alterations in fibroblasts in age-related fibrosis: NADPH oxidase 4 (NOX4) and mitochondrial ROS (mitoROS) have been implicated in myofibroblast differentiation. Peroxisomal dysfunction and/or a deficiency in catalase may exacerbate oxidative stress. Caveolin-1 (Cav-1), a negative regulator of NOX enzymes, is decreased in myofibroblasts. Transforming growth factor-β1 (TGF-β1) and cysteine-rich-61(CYR61)/CCN family member-1 (CCN1) cooperatively induces the oxidative stress in fibroblasts. Deficient antioxidant mechanisms such as nuclear factor erythroid-2 related factor 2 (Nrf2) leads to sustained oxidative stress. The sustained oxidative stress due to decreased antioxidant activity and increased oxidant production promotes pro-fibrotic mesenchymal phenotype. Red arrows indicate direction of change in age-related fibrosis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
4.3. Inflammatory cells
Although the precise role of inflammation in fibrosis is debated [140] and [141] , there is clear evidence for chronic inflammation in fibrotic disorders such as IPF. A number of studies suggest presence of activated inflammatory cells such as alveolar macrophages and neutrophils in IPF lung [142] , [143] , [144] and [145] . These phagocytic cells may contribute to oxidant-mediated epithelial injury [146] and [147] . In addition, IPF lung tissues demonstrate the accumulation of activated non-proliferating T cells and B cells with a dense network of follicular dendritic cells [148] , [149] , [150] and [151] . “Inflammaging” is systemic low grade inflammation associated with aging, accompanied by diminished immunocompetence due to imunosenescence, compromised immunoregulatory mechanisms and/or increased autoimmunity [152] and [153] . Loss of immune regulation with inflammaging may explain, in part, autoantibodies associated with IPF [154] , [155] , [156] , [157] and [158] .
Neutrophils, the most abundant of circulating granulocytes, are part of innate immune system and are recruited during tissue injury. Neutrophils contain NADPH oxidases and myeloperoxidases that generate large quantities of oxidants, O2.− , H2 O2 , and hypochlorous acid (HOCl) [159] and [160] . Neutrophil accumulation and activation have been shown to correlate with disease progression in IPF [147] , [161] and [162] . The contribution of aging to neutrophil superoxide production is less clear, with marked variability depending on the stimulus; some studies suggest that neutrophils have reduced oxidative burst capacity with aging [163] , [164] , [165] and [166] , and other studies suggest normal or increased superoxide production [167] and [168] . Neutrophils isolated from elderly subjects produce lower levels of superoxide in response to N-formyl-methionyl-leucyl-phenylalanine at early time-points, while this effect is sustained over a longer period; this may be related to a failure in the regulatory pathway to de-activate neutrophils in aged individuals [169] . These studies suggest that counter-regulatory mechanisms or intrinsic cellular dysfunction (such as senescence) may result in sustained ROS production, epithelial injury and fibrosis.
Macrophages may mediate both pro- and anti-fibrotic effects depending on its phenotype during the evolution of tissue injury repair. There are two distinct phenotypes of macrophages: M1 polarized macrophages are responsible for initial inflammatory response and clearing of pathogens, whereas M2 polarized macrophages are resolution of inflammation, fighting parasitic infections and tissue remodeling [170] and [171] . Macrophages can promote fibrosis through both direct and indirect actions [172] . Macrophages, especially M2 polarized, can activate myofibroblasts by secreting pro-fibrotic mediators such as TGF-β1 [173] . Arginase, expressed in M2 macrophages, can promote the production of collagen substrates such as proline and glutamate [173] and [174] .
Superoxide is converted to H2 O2 , either spontaneously, or by superoxide dismutases (SODs). Mitochondrial Cu, Zn-SOD expression is increased in alveolar macrophages of asbestosis patients and can promote fibrosis by augmentation of H2 O2 production [175] . Recently, Cu, Zn-SOD (SOD1), has been reported to mediate the M2 polarization of macrophages in redox-dependent manner to promote fibrotic tissue repair [176] and [177] . Macrophage polarization can be affected by aging [178] . The M2 response to interleukin-4 (an M2 inducer) is diminished in aged macrophages, which suggests that diminished M2 responses during aging may contribute to impaired resolution of inflammation [178] . Further studies are required to investigate the effect of aging on macrophage polarization and redox signaling in age-related fibrosis.
Alveolar macrophages isolated from bronchoalveolar lavage of IPF patients produce significantly elevated levels of ROS [179] . High levels of myeloperoxidases in alveolar epithelial lining fluid is associated with increased epithelial injury and rapid decline of IPF patients [147] . In animal model of asbestos-induced fibrosis, alveolar macrophages produce H2 O2 , derived from complex III of the mitochondrial electron transport chain. Rac1 is a member of the Rho family of small GTPases which regulates actin polymerization and ROS generation. Rac1 null mice when asbestos have less oxidative stress and are protected from pulmonary fibrosis [180] . Intra-tracheal exogenous catalase prevents pulmonary fibrosis prevented mice form asbestos induced fibrosis [180] . These studies suggest that macrophages in IPF actively produce ROS and growth factors which can not only lead to epithelial cell damage and mesenchymal activation (Fig. 4 ). During aging, there is increase in population of alveolar macrophages [181] . Older mice also show elevated levels of pro-inflammatory cytokines and a resident population of highly activated pulmonary macrophages [182] . Paradoxically, macrophages from aged mice and rats show diminished capacity of superoxide production [183] , [184] and [185] . As a consequence of reduced respiratory burst, elderly subjects may be more prone to opportunistic bacterial and viral infections [186] and [187] . Human herpes virus (HHV) and Epstein–Barr virus (EBV) have been implicated in the pathogenesis of IPF [188] . Macrophages following herpes virus infection generate increased ROS, which may mediate both antiviral effects and contribute to tissue injury [189] , [190] and [191] . HHV is found in macrophages of IPF lung [192] , suggesting that the virus may evade killing by macrophages. Although there is a decline in phagocytic functions of macrophages with aging, a sustained chronic, low level of inflammation due inflammatory cytokine production by macrophages (known as inflammaging) may contribute to persistent fibrosis with aging [193] . The role of immunosenescence and inflammaging in age-related lung fibrosis deserves further study.
|
|
|
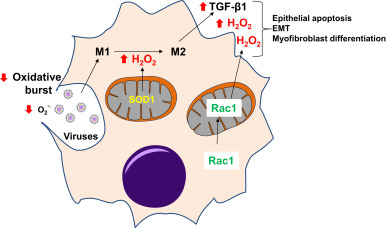
Fig. 4. Redox alterations in macrophages in age-related fibrosis: Deficient oxidative burst in aged macrophages may increase susceptibility to viral infections, which results in sustained inflammatory response and epithelial damage. Hydrogen peroxide (H2 O2 ), catalyzed by superoxide dismutase-1 (SOD1), has been reported to regulate M1 to M2 polarization of macrophages. M2 macrophages secrete pro-fibrotic factors such as transforming growth factor-β1 (TGF-β1) and Rac1-dependent H2 O2 secretion, which further promote pro-fibrotic phenotypes in epithelial cells and fibroblasts. Red arrows indicate direction of change in age-related fibrosis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
5. Potential of redox modulatory therapy in IPF
Aging is accompanied by increase in oxidative modifications of cellular as well as extracellular proteins and these oxidized proteins contribute to pathophysiology of age-related diseases [194] and [195] . Oxidized proteins undergo covalent crosslinking such as dityrosine and disulfide formation and result in the accumulation of non-degradable insoluble protein aggregates [196] and [197] . Fibrosis is characterized by excessive accumulation of insoluble matrix proteins and crosslinking of matrix proteins can hinder the resolution of fibrosis [198] . Our lab has shown that TGF-β1 induces oxidative dityrosine-dependent cross-linking of matrix proteins in lung fibroblasts [199] and that the levels of protein tyrosine oxidation are significantly elevated in plasma of patients with interstitial lung diseases [200] . It is possible that the non-degradation and progressive accumulation of insoluble oxidized proteins can contribute to persistent fibrosis with aging. Further studies are necessary to understand the precise role of these chemical modifications in age-related fibrosis.
Current studies suggest that the homeostatic balance between oxidants and antioxidants is altered in age-related lung fibrosis and contribute to its pathogenesis. Restoration of redox imbalance using exogenous redox modulatory agents could be an effective therapeutic strategy in age-related lung fibrosis. GSH levels are essential for an optimal antioxidant defense against oxidative stress. N-acetylcysteine (NAC), a precursor of GSH synthesis, is a scavenger of ROS and has been shown to ameliorate fibrosis in various animal models [201] and [202] . NAC has been shown to improve redox imbalance in IPF [203] ; however, its therapeutic efficacy is variable with most of studies indicating minimal effect [204] and [205] . Genetic factors such as specific gene polymorphisms may explain the variable response to NAC therapy in IPF patients, and suggest personalized approaches to more effective treatment of IPF [206] . GKT137831, a specific NOX1/4 inhibitor developed by Genkyotex (Geneva, Switzerland), has been shown to partially reverse persistent fibrosis in aged mice [39] . However, its therapeutic efficacy has not been evaluated in IPF patients. Resveratrol, a plant-derived antioxidant stilbenoid, attenuates oxidative stress and fibrosis in animal models [207] and [208] , although clinical trials have not been conducted. Similarly, studies of Nrf2 activators for IPF are lacking. Thus, clinical studies of a number of potentially effective redox-modulatory therapies may be anticipated in the near future. In addition to the identification of critical targets and development of safe, selective compounds for clinical testing, personalized approaches to study design are likely to produce the best results.
6. Conclusion
Normal tissue repair mandates a well-organized temporal execution of reparative functions and interactions between different cell types. Imbalance in redox regulatory mechanisms associated with aging can result from deficient activity of antioxidant mechanisms or elevated generation of ROS. Age-related increases in ROS generation may be related to mitochondrial dysfunction and/or higher expression and activity of NOX enzymes. As highlighted in this review, the redox imbalance with aging can lead to profound alterations in cellular phenotypes and cell-cell interactions that amplify fibrotic responses. Strategies that reinstate redox balance in age-related lung fibrosis may prove to be therapeutically more effective.
Acknowledgements
This work was supported, in whole or in part, by National Institutes of Health Grants, P01 HL114470 and R01 AG046210 , and Veterans Administration Grant, 1I01BX003056 . The authors declare that they have no conflicts of interest with the content of this article.
References
- [1] T.A. Wynn, T.R. Ramalingam; Mechanisms of fibrosis: therapeutic translation for fibrotic disease; Nat. Med., 18 (2012), pp. 1028–1040
- [2] G. Raghu, S.Y. Chen, W.S. Yeh, B. Maroni, Q. Li, Y.C. Lee, H.R. Collard; Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11; Lancet Respir. Med., 2 (2014), pp. 566–572
- [3] L. Nalysnyk, J. Cid-Ruzafa, P. Rotella, D. Esser; Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature; Eur. Respir. Rev., 21 (2012), pp. 355–361
- [4] G. Raghu, D. Weycker, J. Edelsberg, W.Z. Bradford, G. Oster; Incidence and prevalence of idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 174 (2006), pp. 810–816
- [5] A. Biernacka, N.G. Frangogiannis; Aging and cardiac fibrosis; Aging Dis., 2 (2011), pp. 158–173
- [6] N. Kashihara, M. Satoh; Aging and renal fibrosis; Nihon Jinzo Gakkai Shi, 57 (2015), pp. 1206–1214
- [7] H.C. Yang, A.B. Fogo; Fibrosis and renal aging; Kidney Int. Suppl. (2011), 4 (2014), pp. 75–78
- [8] H. Miyaaki, T. Ichikawa, K. Nakao, H. Yatsuhashi, R. Furukawa, K. Ohba, K. Omagari, Y. Kusumoto, K. Yanagi, O. Inoue, N. Kinoshita, H. Ishibashi, M. Yano, K. Eguchi; Clinicopathological study of nonalcoholic fatty liver disease in Japan: the risk factors for fibrosis; Liver Int., 28 (2008), pp. 519–524
- [9] V.J. Thannickal; Mechanistic links between aging and lung fibrosis; Biogerontology, 14 (2013), pp. 609–615
- [10] R.S. Balaban, S. Nemoto, T. Finkel; Mitochondria, oxidants, and aging; Cell, 120 (2005), pp. 483–495
- [11] V.L. Kinnula; Redox imbalance and lung fibrosis; Antioxid. Redox Signal, 10 (2008), pp. 249–252
- [12] V.J. Thannickal, B.L. Fanburg; Reactive oxygen species in cell signaling; Am. J. Physiol. Lung Cell Mol. Physiol., 279 (2000), pp. L1005–L1028
- [13] P. Montuschi, G. Ciabattoni, P. Paredi, P. Pantelidis, R.M. du Bois, S.A. Kharitonov, P.J. Barnes; 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases; Am. J. Respir. Crit. Care Med., 158 (1998), pp. 1524–1527
- [14] I. Rahman, E. Skwarska, M. Henry, M. Davis, C.M. O’Connor, M.X. FitzGerald, A. Greening, W. MacNee; Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis; Free Radic. Biol. Med., 27 (1999), pp. 60–68
- [15] V.L. Kinnula, C.L. Fattman, R.J. Tan, T.D. Oury; Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy; Am. J. Respir. Crit. Care Med., 172 (2005), pp. 417–422
- [16] D.M. Okamura, S. Pennathur; The balance of powers: Redox regulation of fibrogenic pathways in kidney injury; Redox Biol., 6 (2015), pp. 495–504
- [17] E. Crosas-Molist, I. Fabregat; Role of NADPH oxidases in the redox biology of liver fibrosis; Redox Biol., 6 (2015), pp. 106–111
- [18] T. Finkel, N.J. Holbrook; Oxidants, oxidative stress and the biology of ageing; Nature, 408 (2000), pp. 239–247
- [19] C. Lopez-Otin, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer; The hallmarks of aging; Cell, 153 (2013), pp. 1194–1217
- [20] M. Chilosi, A. Carloni, A. Rossi, V. Poletti; Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema; Transl. Res., 162 (2013), pp. 156–173
- [21] A.L. Olson, J.J. Swigris; Idiopathic pulmonary fibrosis: diagnosis and epidemiology; Clin. Chest Med., 33 (2012), pp. 41–50
- [22] H.R. Collard; The age of idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 181 (2010), pp. 771–772
- [23] T.E. King Jr., J.A. Tooze, M.I. Schwarz, K.R. Brown, R.M. Cherniack; Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model; Am. J. Respir. Crit. Care Med., 164 (2001), pp. 1171–1181
- [24] T. Araki, H. Katsura, M. Sawabe, K. Kida; A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients; Intern. Med., 42 (2003), pp. 483–489
- [25] W. Reichel, R. Garcia-Bunuel; Pathologic findings in progeria: myocardial fibrosis and lipofuscin pigment; Am. J. Clin. Pathol., 53 (1970), pp. 243–253
- [26] M.A. Blasco; Telomere length, stem cells and aging; Nat. Chem. Biol., 3 (2007), pp. 640–649
- [27] S.E. Stanley, M. Armanios; Short telomeres: a repeat offender in IPF; Lancet Respir. Med., 2 (2014), pp. 513–514
- [28] M. Armanios; Telomerase and idiopathic pulmonary fibrosis; Mutat. Res., 730 (2012), pp. 52–58
- [29] M.A. Serra, F. Rodriguez, J.A. del Olmo, A. Escudero, J.M. Rodrigo; Influence of age and date of infection on distribution of hepatitis C virus genotypes and fibrosis stage; J. Viral Hepat., 10 (2003), pp. 183–188
- [30] P.D. Ray, B.W. Huang, Y. Tsuji; Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling; Cell Signal, 24 (2012), pp. 981–990
- [31] V.L. Kinnula, M. Myllarniemi; Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis; Antioxid. Redox Signal, 10 (2008), pp. 727–738
- [32] V.J. Thannickal; Aging, antagonistic pleiotropy and fibrotic disease; Int. J. Biochem. Cell Biol., 42 (2010), pp. 1398–1400
- [33] J.D. Lambeth; Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy; Free Radic. Biol. Med., 43 (2007), pp. 332–347
- [34] J.S. Duffield, M. Lupher, V.J. Thannickal, T.A. Wynn; Host responses in tissue repair and fibrosis; Annu Rev. Pathol., 8 (2013), pp. 241–276
- [35] V. Krizhanovsky, M. Yon, R.A. Dickins, S. Hearn, J. Simon, C. Miething, H. Yee, L. Zender, S.W. Lowe; Senescence of activated stellate cells limits liver fibrosis; Cell, 134 (2008), pp. 657–667
- [36] J.I. Jun, L.F. Lau; The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing; Nat. Cell Biol., 12 (2010), pp. 676–685
- [37] D.R. Waisberg, J.V. Barbas-Filho, E.R. Parra, S. Fernezlian, C.R. de Carvalho, R.A. Kairalla, V.L. Capelozzi; Abnormal expression of telomerase/apoptosis limits type II alveolar epithelial cell replication in the early remodeling of usual interstitial pneumonia/idiopathic pulmonary fibrosis; Hum. Pathol., 41 (2010), pp. 385–391
- [38] B. Driscoll, S. Buckley, K.C. Bui, K.D. Anderson, D. Warburton; Telomerase in alveolar epithelial development and repair; Am. J. Physiol. Lung Cell Mol. Physiol., 279 (2000), pp. L1191–L1198
- [39] L. Hecker, N.J. Logsdon, D. Kurundkar, A. Kurundkar, K. Bernard, T. Hock, E. Meldrum, Y.Y. Sanders, V.J. Thannickal; Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance; Sci. Transl. Med., 6 (2014) 231ra247
- [40] W.T. Huang, H. Akhter, C. Jiang, M. MacEwen, Q. Ding, V. Antony, V.J. Thannickal, R.M. Liu; Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis; Exp. Gerontol., 61 (2015), pp. 62–75
- [41] D.P. Jones; Redox theory of aging; Redox Biol., 5 (2015), pp. 71–79
- [42] A. Covarrubias, V. Byles, T. Horng; ROS sets the stage for macrophage differentiation; Cell Res., 23 (2013), pp. 984–985
- [43] L. Hecker, R. Vittal, T. Jones, R. Jagirdar, T.R. Luckhardt, J.C. Horowitz, S. Pennathur, F.J. Martinez, V.J. Thannickal; NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury; Nat. Med., 15 (2009), pp. 1077–1081
- [44] E. Borkham-Kamphorst, C. Schaffrath, E. Van de Leur, U. Haas, L. Tihaa, S.K. Meurer, Y.A. Nevzorova, C. Liedtke, R. Weiskirchen; The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling; Biochim. Biophys. Acta, 1843 (2014), pp. 902–914
- [45] E. Novo, C. Busletta, L.V. Bonzo, D. Povero, C. Paternostro, K. Mareschi, I. Ferrero, E. David, C. Bertolani, A. Caligiuri, S. Cannito, E. Tamagno, A. Compagnone, S. Colombatto, F. Marra, F. Fagioli, M. Pinzani, M. Parola; Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells; J. Hepatol., 54 (2011), pp. 964–974
- [46] Y. Meng, T. Li, G.S. Zhou, Y. Chen, C.H. Yu, M.X. Pang, W. Li, Y. Li, W.Y. Zhang, X. Li; The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway; Antioxid. Redox Signal, 22 (2015), pp. 241–258
- [47] M.L. Taddei, E. Giannoni, G. Raugei, S. Scacco, A.M. Sardanelli, S. Papa, P. Chiarugi; Mitochondrial Oxidative Stress due to Complex I Dysfunction Promotes Fibroblast Activation and Melanoma Cell Invasiveness; J. Signal Transduct., 2012 (2012), p. 684592
- [48] L. Hecker, V.J. Thannickal; Getting to the core of fibrosis: targeting redox imbalance in aging; Ann. Transl. Med., 4 (2016), p. 93
- [49] M. Selman, A. Pardo; Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers; Proc. Am. Thorac. Soc., 3 (2006), pp. 364–372
- [50] M. Plataki, A.V. Koutsopoulos, K. Darivianaki, G. Delides, N.M. Siafakas, D. Bouros; Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis; Chest, 127 (2005), pp. 266–274
- [51] V.J. Thannickal, J.C. Horowitz; Evolving concepts of apoptosis in idiopathic pulmonary fibrosis; Proc. Am. Thorac. Soc., 3 (2006), pp. 350–356
- [52] K. Kuwano, R. Kunitake, M. Kawasaki, Y. Nomoto, N. Hagimoto, Y. Nakanishi, N. Hara; P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 154 (1996), pp. 477–483
- [53] S. Minagawa, J. Araya, T. Numata, S. Nojiri, H. Hara, Y. Yumino, M. Kawaishi, M. Odaka, T. Morikawa, S.L. Nishimura, K. Nakayama, K. Kuwano; Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells; Am. J. Physiol. Lung Cell Mol. Physiol., 300 (2011), pp. L391–L401
- [54] N. Khalil, R.N. O’Connor, K.C. Flanders, H. Unruh; TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study; Am. J. Respir. Cell Mol. Biol., 14 (1996), pp. 131–138
- [55] H.N. Antoniades, M.A. Bravo, R.E. Avila, T. Galanopoulos, J. Neville-Golden, M. Maxwell, M. Selman; Platelet-derived growth factor in idiopathic pulmonary fibrosis; J. Clin. Investig., 86 (1990), pp. 1055–1064
- [56] A. Giaid, R.P. Michel, D.J. Stewart, M. Sheppard, B. Corrin, Q. Hamid; Expression of endo thelin-1 in lungs of patients with cryptogenic fibrosing alveolitis ; Lancet, 341 (1993), pp. 1550–1554
- [57] X. Li, M. Molina-Molina, A. Abdul-Hafez, J. Ramirez, A. Serrano-Mollar, A. Xaubet, B.D. Uhal; Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis; Am. J. Physiol. Lung Cell Mol. Physiol., 291 (2006), pp. L887–L895
- [58] L.H. Pan, K. Yamauchi, M. Uzuki, T. Nakanishi, M. Takigawa, H. Inoue, T. Sawai; Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF; Eur. Respir. J., 17 (2001), pp. 1220–1227
- [59] N. Hagimoto, K. Kuwano, H. Miyazaki, R. Kunitake, M. Fujita, M. Kawasaki, Y. Kaneko, N. Hara; Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen; Am. J. Respir. Cell Mol. Biol., 17 (1997), pp. 272–278
- [60] K. Kuwano, N. Hagimoto, M. Kawasaki, T. Yatomi, N. Nakamura, S. Nagata, T. Suda, R. Kunitake, T. Maeyama, H. Miyazaki, N. Hara; Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis; J. Clin. Investig., 104 (1999), pp. 13–19
- [61] J.R. Rock, C.E. Barkauskas, M.J. Cronce, Y. Xue, J.R. Harris, J. Liang, P.W. Noble, B.L. Hogan; Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition; Proc. Natl. Acad. Sci. USA, 108 (2011), pp. E1475–E1483
- [62] B.C. Willis, J.M. Liebler, K. Luby-Phelps, A.G. Nicholson, E.D. Crandall, R.M. du Bois, Z. Borok; Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis; Am. J. Pathol., 166 (2005), pp. 1321–1332
- [63] H. Kage, Z. Borok; EMT and interstitial lung disease: a mysterious relationship; Curr. Opin. Pulm. Med., 18 (2012), pp. 517–523
- [64] M.T. Lin, M.F. Beal; Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases; Nature, 443 (2006), pp. 787–795
- [65] K. Kuwano, N. Nakashima, I. Inoshima, N. Hagimoto, M. Fujita, M. Yoshimi, T. Maeyama, N. Hamada, K. Watanabe, N. Hara; Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias; Eur. Respir. J., 21 (2003), pp. 232–240
- [66] H. Hara, J. Araya, S. Ito, K. Kobayashi, N. Takasaka, Y. Yoshii, H. Wakui, J. Kojima, K. Shimizu, T. Numata, M. Kawaishi, N. Kamiya, M. Odaka, T. Morikawa, Y. Kaneko, K. Nakayama, K. Kuwano; Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence; Am. J. Physiol. Lung Cell Mol. Physiol., 305 (2013), pp. L737–L746
- [67] G. Zhou, L.A. Dada, M. Wu, A. Kelly, H. Trejo, Q. Zhou, J. Varga, J.I. Sznajder; Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1; Am. J. Physiol. Lung Cell Mol. Physiol., 297 (2009), pp. L1120–L1130
- [68] K. Richter, A. Konzack, T. Pihlajaniemi, R. Heljasvaara, T. Kietzmann; Redox-fibrosis: impact of TGFbeta1 on ROS generators, mediators and functional consequences; Redox Biol., 6 (2015), pp. 344–352
- [69] T.J. Broekelmann, A.H. Limper, T.V. Colby, J.A. McDonald; Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis; Proc. Natl. Acad. Sci. USA, 88 (1991), pp. 6642–6646
- [70] N. Hagimoto, K. Kuwano, I. Inoshima, M. Yoshimi, N. Nakamura, M. Fujita, T. Maeyama, N. Hara; TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells; J. Immunol., 168 (2002), pp. 6470–6478
- [71] K. Yanagisawa, H. Osada, A. Masuda, M. Kondo, T. Saito, Y. Yatabe, K. Takagi, T. Takahashi, T. Takahashi; Induction of apoptosis by Smad3 and down-regulation of Smad3 expression in response to TGF-beta in human normal lung epithelial cells; Oncogene, 17 (1998), pp. 1743–1747
- [72] D.Y. Rhyu, Y. Yang, H. Ha, G.T. Lee, J.S. Song, S.T. Uh, H.B. Lee; Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells; J. Am. Soc. Nephrol., 16 (2005), pp. 667–675
- [73] Y.S. Yoon, J.H. Lee, S.C. Hwang, K.S. Choi, G. Yoon; TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells; Oncogene, 24 (2005), pp. 1895–1903
- [74] E.E. Jazin, L. Cavelier, I. Eriksson, L. Oreland, U. Gyllensten; Human brain contains high levels of heteroplasmy in the noncoding regions of mitochondrial DNA; Proc. Natl. Acad. Sci. USA, 93 (1996), pp. 12382–12387
- [75] M.T. Lin, D.K. Simon, C.H. Ahn, L.M. Kim, M.F. Beal; High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain; Hum. Mol. Genet, 11 (2002), pp. 133–145
- [76] M. Bueno, Y.C. Lai, Y. Romero, J. Brands St, C.M. Croix, C. Kamga, C. Corey, J.D. Herazo-Maya, J. Sembrat, J.S. Lee, S.R. Duncan, M. Rojas, S. Shiva, C.T. Chu, A.L. Mora; PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis; J. Clin. Investig., 125 (2015), pp. 521–538
- [77] J.H. Santos, L. Hunakova, Y. Chen, C. Bortner, B. Van Houten; Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death; J. Biol. Chem., 278 (2003), pp. 1728–1734
- [78] D.R. Green, G. Kroemer; The pathophysiology of mitochondrial cell death; Science, 305 (2004), pp. 626–629
- [79] C.A. Gautier, T. Kitada, J. Shen; Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress; Proc. Natl. Acad. Sci. USA, 105 (2008), pp. 11364–11369
- [80] J.W. Pridgeon, J.A. Olzmann, L.S. Chin, L. Li; PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1; PLoS Biol., 5 (2007), p. e172
- [81] J.A. Shin, J.S. Chung, S.H. Cho, H.J. Kim, Y.D. Yoo; Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells; Biochem. Biophys. Res. Commun., 439 (2013), pp. 315–320
- [82] Y.M. Chung, S.B. Lee, H.J. Kim, S.H. Park, J.J. Kim, J.S. Chung, Y.D. Yoo; Replicative senescence induced by Romo1-derived reactive oxygen species; J. Biol. Chem., 283 (2008), pp. 33763–33771
- [83] H. Zou, E. Stoppani, D. Volonte, F. Galbiati; Caveolin-1, cellular senescence and age-related diseases; Mech. Ageing Dev., 132 (2011), pp. 533–542
- [84] D. Volonte, F. Galbiati; Caveolin-1, cellular senescence and pulmonary emphysema; Aging, 1 (2009), pp. 831–835
- [85] M.J. Kang, Y.H. Chung, C.I. Hwang, M. Murata, T. Fujimoto, I.H. Mook-Jung, C.I. Cha, W.Y. Park; Caveolin-1 upregulation in senescent neurons alters amyloid precursor protein processing; Exp. Mol. Med., 38 (2006), pp. 126–133
- [86] Y.Y. Sanders, H. Liu, X. Zhang, L. Hecker, K. Bernard, L. Desai, G. Liu, V.J. Thannickal; Histone modifications in senescence-associated resistance to apoptosis by oxidative stress; Redox Biol., 1 (2013), pp. 8–16
- [87] A. Dasari, J.N. Bartholomew, D. Volonte, F. Galbiati; Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements; Cancer Res., 66 (2006), pp. 10805–10814
- [88] P. Shivshankar, C. Brampton, S. Miyasato, M. Kasper, V.J. Thannickal, C.J. Le Saux; Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice; Am. J. Respir. Cell Mol. Biol., 47 (2012), pp. 28–36
- [89] M.V. Gattas, R. Forteza, M.A. Fragoso, N. Fregien, P. Salas, M. Salathe, G.E. Conner; Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli; Free Radic. Biol. Med., 47 (2009), pp. 1450–1458
- [90] R. Forteza, M. Salathe, F. Miot, R. Forteza, G.E. Conner; Regulated hydrogen peroxide production by Duox in human airway epithelial cells; Am. J. Respir. Cell Mol. Biol., 32 (2005), pp. 462–469
- [91] S. Carnesecchi, C. Deffert, Y. Donati, O. Basset, B. Hinz, O. Preynat-Seauve, C. Guichard, J.L. Arbiser, B. Banfi, J.C. Pache, C. Barazzone-Argiroffo, K.H. Krause; A key role for NOX4 in epithelial cell death during development of lung fibrosis; Antioxid. Redox Signal, 15 (2011), pp. 607–619
- [92] N.M. Reddy, S.R. Kleeberger, H.Y. Cho, M. Yamamoto, T.W. Kensler, S. Biswal, S.P. Reddy; Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants; Am. J. Respir. Cell Mol. Biol., 37 (2007), pp. 3–8
- [93] H.Y. Cho, S.P. Reddy, M. Yamamoto, S.R. Kleeberger; The transcription factor NRF2 protects against pulmonary fibrosis; FASEB J., 18 (2004), pp. 1258–1260
- [94] K. Tomobe, T. Shinozuka, M. Kuroiwa, Y. Nomura; Age-related changes of Nrf2 and phosphorylated GSK-3beta in a mouse model of accelerated aging (SAMP8); Arch. Gerontol. Geriatr., 54 (2012), pp. e1–e7
- [95] H. Zhang, K.J. Davies, H.J. Forman; Oxidative stress response and Nrf2 signaling in aging; Free Radic. Biol. Med., 88 (2015), pp. 314–336
- [96] Z. Ungvari, L. Bailey-Downs, D. Sosnowska, T. Gautam, P. Koncz, G. Losonczy, P. Ballabh, R. de Cabo, W.E. Sonntag, A. Csiszar; Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response; Am. J. Physiol. Heart Circ. Physiol., 301 (2011), pp. H363–H372
- [97] K.M. Beeh, J. Beier, I.C. Haas, O. Kornmann, P. Micke, R. Buhl; Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis; Eur. Respir. J., 19 (2002), pp. 1119–1123
- [98] A.M. Cantin, R.C. Hubbard, R.G. Crystal; Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis; Am. Rev. Respir. Dis., 139 (1989), pp. 370–372
- [99] H. Jardine, W. MacNee, K. Donaldson, I. Rahman; Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1; J. Biol. Chem., 277 (2002), pp. 21158–21166
- [100] K. Arsalane, C.M. Dubois, T. Muanza, R. Begin, F. Boudreau, C. Asselin, A.M. Cantin; Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase; Am. J. Respir. Cell Mol. Biol., 17 (1997), pp. 599–607
- [101] R.M. Liu, P.K. Vayalil, C. Ballinger, D.A. Dickinson, W.T. Huang, S. Wang, T.J. Kavanagh, Q.L. Matthews, E.M. Postlethwait; Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model; Free Radic. Biol. Med., 53 (2012), pp. 554–563
- [102] N.S. Gould, E. Min, S. Gauthier, H.W. Chu, R. Martin, B.J. Day; Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung; Am. J. Respir. Crit. Care Med., 182 (2010), pp. 1114–1122
- [103] J. Lu, A. Holmgren; The thioredoxin antioxidant system; Free Radic. Biol. Med., 66 (2014), pp. 75–87
- [104] L. Tiitto, R. Kaarteenaho-Wiik, R. Sormunen, A. Holmgren, P. Paakko, Y. Soini, V.L. Kinnula; Expression of the thioredoxin system in interstitial lung disease; J. Pathol., 201 (2003), pp. 363–370
- [105] E. Lakari, P. Paakko, P. Pietarinen-Runtti, V.L. Kinnula; Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung; Am. J. Respir. Crit. Care Med., 161 (2000), pp. 615–621
- [106] V.J. Thannickal, G.B. Toews, E.S. White, J.P. Lynch 3rd, F.J. Martinez; Mechanisms of pulmonary fibrosis; Annu Rev. Med., 55 (2004), pp. 395–417
- [107] B. Hinz, S.H. Phan, V.J. Thannickal, A. Galli, M.L. Bochaton-Piallat, G. Gabbiani; The myofibroblast: one function, multiple origins; Am. J. Pathol., 170 (2007), pp. 1807–1816
- [108] T.E. King Jr., M.I. Schwarz, K. Brown, J.A. Tooze, T.V. Colby, J.A. Waldron Jr., A. Flint, W. Thurlbeck, R.M. Cherniack; Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality.; Am. J. Respir. Crit. Care Med., 164 (2001), pp. 1025–1032
- [109] L. Tiitto, R. Bloigu, U. Heiskanen, P. Paakko, V.L. Kinnula, R. Kaarteenaho-Wiik; Relationship between histopathological features and the course of idiopathic pulmonary fibrosis/usual interstitial pneumonia; Thorax, 61 (2006), pp. 1091–1095
- [110] A.G. Nicholson, L.G. Fulford, T.V. Colby, R.M. du Bois, D.M. Hansell, A.U. Wells; The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 166 (2002), pp. 173–177
- [111] E.S. White, M.H. Lazar, V.J. Thannickal; Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis; J. Pathol., 201 (2003), pp. 343–354
- [112] H. Yanai, A. Shteinberg, Z. Porat, A. Budovsky, A. Braiman, R. Zeische, V.E. Fraifeld; Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients; Aging, 7 (2015), pp. 664–672
- [113] H.C. Lee, P.H. Yin, C.W. Chi, Y.H. Wei; Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence; J. Biomed. Sci., 9 (2002), pp. 517–526
- [114] M. Jain, S. Rivera, E.A. Monclus, L. Synenki, A. Zirk, J. Eisenbart, C. Feghali-Bostwick, G.M. Mutlu, G.R. Budinger, N.S. Chandel; Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling; J. Biol. Chem., 288 (2013), pp. 770–777
- [115] C.C. Chen, J.L. Young, R.I. Monzon, N. Chen, V. Todorovic, L.F. Lau; Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling; EMBO J., 26 (2007), pp. 1257–1267
- [116] A.R. Kurundkar, D. Kurundkar, S. Rangarajan, M.L. Locy, Y. Zhou, R.M. Liu, J. Zmijewski, V.J. Thannickal; The matricellular protein CCN1 enhances TGF-beta1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury; FASEB J. (2016)
- [117] T. Quan, Z. Qin, J.J. Voorhees, G.J. Fisher; Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts; J. Cell Biochem., 113 (2012), pp. 3011–3018
- [118] J. Du, J.D. Klein, F. Hassounah, J. Zhang, C. Zhang, X.H. Wang; Aging increases CCN1 expression leading to muscle senescence; Am. J. Physiol. Cell Physiol., 306 (2014), pp. C28–C36
- [119] T. Quan, Z. Qin, P. Robichaud, J.J. Voorhees, G.J. Fisher; CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts; J. Cell Commun. Signal, 5 (2011), pp. 201–207
- [120] M. Schrader, H.D. Fahimi; Peroxisomes and oxidative stress; Biochim Biophys. Acta, 1763 (2006), pp. 1755–1766
- [121] G. Oruqaj, S. Karnati, V. Vijayan, L.K. Kotarkonda, E. Boateng, W. Zhang, C. Ruppert, A. Gunther, W. Shi, E. Baumgart-Vogt; Compromised peroxisomes in idiopathic pulmonary fibrosis, a vicious cycle inducing a higher fibrotic response via TGF-beta signaling; Proc. Natl. Acad. Sci. USA, 112 (2015), pp. E2048–E2057
- [122] J.E. Legakis, J.I. Koepke, C. Jedeszko, F. Barlaskar, L.J. Terlecky, H.J. Edwards, P.A. Walton, S.R. Terlecky; Peroxisome senescence in human fibroblasts; Mol. Biol. Cell, 13 (2002), pp. 4243–4255
- [123] L. Goth, J.W. Eaton; Hereditary catalase deficiencies and increased risk of diabetes; Lancet, 356 (2000), pp. 1820–1821
- [124] O. Yildirim, N.A. Ates, L. Tamer, N. Muslu, B. Ercan, U. Atik, A. Kanik; Changes in antioxidant enzyme activity and malondialdehyde level in patients with age-related macular degeneration; Ophthalmologica, 218 (2004), pp. 202–206
- [125] X.M. Wang, Y. Zhang, H.P. Kim, Z. Zhou, C.A. Feghali-Bostwick, F. Liu, E. Ifedigbo, X. Xu, T.D. Oury, N. Kaminski, A.M. Choi; Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis; J. Exp. Med., 203 (2006), pp. 2895–2906
- [126] Y.Y. Sanders, Z. Cui, C.J. Le Saux, J.C. Horowitz, S. Rangarajan, A. Kurundkar, V.B. Antony, V.J. Thannickal; SMAD-independent down-regulation of caveolin-1 by TGF-beta: effects on proliferation and survival of myofibroblasts; PLoS One, 10 (2015), p. e0116995
- [127] F. Chen, S. Barman, Y. Yu, S. Haigh, Y. Wang, S.M. Black, R. Rafikov, H. Dou, Z. Bagi, W. Han, Y. Su, D.J. Fulton; Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species; Free Radic. Biol. Med., 73 (2014), pp. 201–213
- [128] Y.Y. Sanders, H. Liu, G. Liu, V.J. Thannickal; Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence; Free Radic. Biol. Med., 79 (2015), pp. 197–205
- [129] T. Ago, S. Matsushima, J. Kuroda, D. Zablocki, T. Kitazono, J. Sadoshima; The NADPH oxidase Nox4 and aging in the heart; Aging, 2 (2010), pp. 1012–1016
- [130] D.J. McCrann, D. Yang, H. Chen, S. Carroll, K. Ravid; Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy; Cell Cycle, 8 (2009), pp. 902–908
- [131] N. Amara, D. Goven, F. Prost, R. Muloway, B. Crestani, J. Boczkowski; NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts; Thorax, 65 (2010), pp. 733–738
- [132] T.W. Kensler, N. Wakabayashi, S. Biswal; Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway; Annu Rev. Pharmacol. Toxicol., 47 (2007), pp. 89–116
- [133] H.Y. Cho, S.P. Reddy, S.R. Kleeberger; Nrf2 defends the lung from oxidative stress; Antioxid. Redox Signal, 8 (2006), pp. 76–87
- [134] N. Kikuchi, Y. Ishii, Y. Morishima, Y. Yageta, N. Haraguchi, K. Itoh, M. Yamamoto, N. Hizawa; Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance; Respir. Res., 11 (2010), p. 31
- [135] E. Artaud-Macari, D. Goven, S. Brayer, A. Hamimi, V. Besnard, J. Marchal-Somme, Z.E. Ali, B. Crestani, S. Kerdine-Romer, A. Boutten, M. Bonay; Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis; Antioxid. Redox Signal, 18 (2013), pp. 66–79
- [136] H.A. Chapman; Epithelial-mesenchymal interactions in pulmonary fibrosis; Annu Rev. Physiol., 73 (2011), pp. 413–435
- [137] J.C. Horowitz, V.J. Thannickal; Epithelial-mesenchymal interactions in pulmonary fibrosis; Semin Respir. Crit. Care Med., 27 (2006), pp. 600–612
- [138] B.D. Uhal, I. Joshi, W.F. Hughes, C. Ramos, A. Pardo, M. Selman; Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung; Am. J. Physiol., 275 (1998), pp. L1192–L1199
- [139] M. Waghray, Z. Cui, J.C. Horowitz, I.M. Subramanian, F.J. Martinez, G.B. Toews, V.J. Thannickal; Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts; FASEB J., 19 (2005), pp. 854–856
- [140] R.M. Strieter; Con: Inflammatory mechanisms are not a minor component of the pathogenesis of idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 165 (2002), pp. 1207–1208
- [141] J. Gauldie; Pro: Inflammatory mechanisms are a minor component of the pathogenesis of idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 165 (2002), pp. 1205–1206
- [142] J.D. Fulmer, W.C. Roberts, E.R. von Gal, R.G. Crystal; Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis; J. Clin. Investig., 63 (1979), pp. 665–676
- [143] R.G. Crystal, J.D. Fulmer, B.J. Baum, J. Bernardo, K.H. Bradley, S.D. Bruel, N.A. Elson, G.A. Fells, V.J. Ferrans, J.E. Gadek, G.W. Hunninghake, O. Kawanami, J.A. Kelman, B.R. Line, J.A. McDonald, B.D. McLees, W.C. Roberts, D.M. Rosenberg, P. Tolstoshev, E. Von Gal, S.E. Weinberger; Cells, collagen and idiopathic pulmonary fibrosis; Lung, 155 (1978), pp. 199–224
- [144] J.D. Fulmer, W.C. Roberts, E.R. von Gal, R.G. Grystal; Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations; J. Clin. Investig., 60 (1977), pp. 595–610
- [145] A. Adhyatmika, K.S. Putri, L. Beljaars, B.N. Melgert; The elusive antifibrotic macrophage; Front. Med., 2 (2015), p. 81
- [146] C.F. Nathan, L.H. Brukner, S.C. Silverstein, Z.A. Cohn; Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide; J. Exp. Med., 149 (1979), pp. 84–99
- [147] A.M. Cantin, S.L. North, G.A. Fells, R.C. Hubbard, R.G. Crystal; Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis; J. Clin. Investig., 79 (1987), pp. 1665–1673
- [148] J. Marchal-Somme, Y. Uzunhan, S. Marchand-Adam, D. Valeyre, V. Soumelis, B. Crestani, P. Soler; Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis; J. Immunol., 176 (2006), pp. 5735–5739
- [149] J. Marchal-Somme, Y. Uzunhan, S. Marchand-Adam, M. Kambouchner, D. Valeyre, B. Crestani, P. Soler; Dendritic cells accumulate in human fibrotic interstitial lung disease; Am. J. Respir. Crit. Care Med., 176 (2007), pp. 1007–1014
- [150] G.J. Nuovo, J.S. Hagood, C.M. Magro, N. Chin, R. Kapil, L. Davis, C.B. Marsh, V.A. Folcik; The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis; Mod. Pathol., 25 (2012), pp. 416–433
- [151] J. Xue, D.J. Kass, J. Bon, L. Vuga, J. Tan, E. Csizmadia, L. Otterbein, M. Soejima, M.C. Levesque, K.F. Gibson, N. Kaminski, J.M. Pilewski, M. Donahoe, F.C. Sciurba, S.R. Duncan; Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients; J. Immunol., 191 (2013), pp. 2089–2095
- [152] C. Franceschi; Inflammaging as a major characteristic of old people: can it be prevented or cured?; Nutr. Rev., 65 (2007), pp. S173–S176
- [153] F.T. Hakim, R.E. Gress; Immunosenescence: deficits in adaptive immunity in the elderly; Tissue Antigens, 70 (2007), pp. 179–189
- [154] A.K. Shum, M. Alimohammadi, C.L. Tan, M.H. Cheng, T.C. Metzger, C.S. Law, W. Lwin, J. Perheentupa, H. Bour-Jordan, J.C. Carel, E.S. Husebye, F. De Luca, C. Janson, R. Sargur, N. Dubois, M. Kajosaari, P.J. Wolters, H.A. Chapman, O. Kampe, M.S. Anderson; BPIFB1 is a lung-specific autoantigen associated with interstitial lung disease; Sci. Transl. Med., 5 (2013) 206ra139
- [155] C.A. Feghali-Bostwick, C.G. Tsai, V.G. Valentine, S. Kantrow, M.W. Stoner, J.M. Pilewski, A. Gadgil, M.P. George, K.F. Gibson, A.M. Choi, N. Kaminski, Y. Zhang, S.R. Duncan; Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis; J. Immunol., 179 (2007), pp. 2592–2599
- [156] R.A. Kahloon, J. Xue, A. Bhargava, E. Csizmadia, L. Otterbein, D.J. Kass, J. Bon, M. Soejima, M.C. Levesque, K.O. Lindell, K.F. Gibson, N. Kaminski, G. Banga, C.V. Oddis, J.M. Pilewski, F.C. Sciurba, M. Donahoe, Y. Zhang, S.R. Duncan; Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses; Am. J. Respir. Crit. Care Med., 187 (2013), pp. 768–775
- [157] C. Taille, S. Grootenboer-Mignot, C. Boursier, L. Michel, M.P. Debray, J. Fagart, L. Barrientos, A. Mailleux, N. Cigna, F. Tubach, J. Marchal-Somme, P. Soler, S. Chollet-Martin, B. Crestani; Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis; Am. J. Respir. Crit. Care Med., 183 (2011), pp. 759–766
- [158] K. Kurosu, Y. Takiguchi, O. Okada, N. Yumoto, S. Sakao, Y. Tada, Y. Kasahara, N. Tanabe, K. Tatsumi, M. Weiden, W.N. Rom, T. Kuriyama; Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis; J. Immunol., 181 (2008), pp. 756–767
- [159] J.P. Gaut, G.C. Yeh, H.D. Tran, J. Byun, J.P. Henderson, G.M. Richter, M.L. Brennan, A.J. Lusis, A. Belaaouaj, R.S. Hotchkiss, J.W. Heinecke; Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis; Proc. Natl. Acad. Sci. USA, 98 (2001), pp. 11961–11966
- [160] M.M. Anderson, S.L. Hazen, F.F. Hsu, J.W. Heinecke; Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation; J. Clin. Investig., 99 (1997), pp. 424–432
- [161] G.W. Hunninghake, J.E. Gadek, T.J. Lawley, R.G. Crystal; Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis; J. Clin. Investig., 68 (1981), pp. 259–269
- [162] H.A. Jones, J.B. Schofield, T. Krausz, A.R. Boobis, C. Haslett; Pulmonary fibrosis correlates with duration of tissue neutrophil activation; Am. J. Respir. Crit. Care Med., 158 (1998), pp. 620–628
- [163] P. Angelis, S. Scharf, N. Christophidis; Effects of age on neutrophil function and its relevance to bacterial infections in the elderly; J. Clin. Lab. Immunol., 49 (1997), pp. 33–40
- [164] P.C. Braga, M.T. Sala, M. Dal Sasso, A. Pecile, G. Annoni, C. Vergani; Age-associated differences in neutrophil oxidative burst (chemiluminescence); Exp. Gerontol., 33 (1998), pp. 477–484
- [165] C. Wenisch, S. Patruta, F. Daxbock, R. Krause, W. Horl; Effect of age on human neutrophil function; J. Leukoc. Biol., 67 (2000), pp. 40–45
- [166] D. Sauce, Y. Dong, L. Campillo-Gimenez, S. Casulli, C. Bayard, B. Autran, J. Boddaert, V. Appay, C. Elbim; Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset; J. Gerontol. A Biol. Sci. Med. Sci. (2016)
- [167] J.X. Corberand, P.F. Laharrague, G. Fillola; Neutrophils of healthy aged humans are normal; Mech. Ageing Dev., 36 (1986), pp. 57–63
- [168] B. Esparza, H. Sanchez, M. Ruiz, M. Barranquero, E. Sabino, F. Merino; Neutrophil function in elderly persons assessed by flow cytometry; Immunol. Investig., 25 (1996), pp. 185–190
- [169] T. Fulop, A. Larbi, N. Douziech, C. Fortin, K.P. Guerard, O. Lesur, A. Khalil, G. Dupuis; Signal transduction and functional changes in neutrophils with aging; Aging Cell, 3 (2004), pp. 217–226
- [170] T.A. Wynn, L. Barron; Macrophages: master regulators of inflammation and fibrosis; Semin Liver Dis., 30 (2010), pp. 245–257
- [171] T.A. Wynn, K.M. Vannella; Macrophages in tissue repair, regeneration, and fibrosis; Immunity, 44 (2016), pp. 450–462
- [172] E. Hams, R. Bermingham, P.G. Fallon; Macrophage and innate lymphoid cell interplay in the genesis of fibrosis; Front Immunol., 6 (2015), p. 597
- [173] L. Barron, T.A. Wynn; Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages; Am. J. Physiol. Gastrointest. Liver Physiol., 300 (2011), pp. G723–G728
- [174] M.A. Gibbons, A.C. MacKinnon, P. Ramachandran, K. Dhaliwal, R. Duffin, A.T. Phythian-Adams, N. van Rooijen, C. Haslett, S.E. Howie, A.J. Simpson, N. Hirani, J. Gauldie, J.P. Iredale, T. Sethi, S.J. Forbes; Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis; Am. J. Respir. Crit. Care Med., 184 (2011), pp. 569–581
- [175] C. He, S. Murthy, M.L. McCormick, D.R. Spitz, A.J. Ryan, A.B. Carter; Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2 O2 generation ; J. Biol. Chem., 286 (2011), pp. 15597–15607
- [176] C. He, A.J. Ryan, S. Murthy, A.B. Carter; Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages; J. Biol. Chem., 288 (2013), pp. 20745–20757
- [177] C. He, J.L. Larson-Casey, L. Gu, A.J. Ryan, S. Murthy, A.B. Carter; Cu,Zn-SOD-mediated Redox regulation of Jmjd3 modulates macrophage polarization and pulmonary fibrosis; Am. J. Respir. Cell Mol. Biol. (2015)
- [178] S. Mahbub, C.R. Deburghgraeve, E.J. Kovacs; Advanced age impairs macrophage polarization; J. Interferon Cytokine Res., 32 (2012), pp. 18–26
- [179] J. Kiemle-Kallee, H. Kreipe, H.J. Radzun, M.R. Parwaresch, U. Auerswald, H. Magnussen, J. Barth; Alveolar macrophages in idiopathic pulmonary fibrosis display a more monocyte-like immunophenotype and an increased release of free oxygen radicals; Eur. Respir. J., 4 (1991), pp. 400–406
- [180] S. Murthy, A. Adamcakova-Dodd, S.S. Perry, L.A. Tephly, R.M. Keller, N. Metwali, D.K. Meyerholz, Y. Wang, M. Glogauer, P.S. Thorne, A.B. Carter; Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis; Am. J. Physiol. Lung Cell Mol. Physiol., 297 (2009), pp. L846–L855
- [181] W.A. Wallace, M. Gillooly, D. Lamb; Age related increase in the intra-alveolar macrophage population of non-smokers; Thorax, 48 (1993), pp. 668–669
- [182] C.H. Canan, N.S. Gokhale, B. Carruthers, W.P. Lafuse, L.S. Schlesinger, J.B. Torrelles, J. Turner; Characterization of lung inflammation and its impact on macrophage function in aging; J. Leukoc. Biol., 96 (2014), pp. 473–480
- [183] L. Lavie, O. Weinreb, D. Gershon; Age-related alterations in superoxide anion generation in mouse peritoneal macrophages studied by repeated stimulations and heat shock treatment; J. Cell Physiol., 152 (1992), pp. 382–388
- [184] H. Hayakawa, A. Sato, T. Yagi, H. Uchiyama, K. Ide, M. Nakano; Superoxide generation by alveolar macrophages from aged rats: improvement by in vitro treatment with IFN-gamma; Mech. Ageing Dev., 80 (1995), pp. 199–211
- [185] D.R. Tasat, R. Mancuso, S. O’Connor, B. Molinari; Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages; Aging Cell, 2 (2003), pp. 159–164
- [186] A. Panda, A. Arjona, E. Sapey, F. Bai, E. Fikrig, R.R. Montgomery, J.M. Lord, A.C. Shaw; Human innate immunosenescence: causes and consequences for immunity in old age; Trends Immunol., 30 (2009), pp. 325–333
- [187] H.Y. Chung, M. Cesari, S. Anton, E. Marzetti, S. Giovannini, A.Y. Seo, C. Carter, B.P. Yu, C. Leeuwenburgh; Molecular inflammation: underpinnings of aging and age-related diseases; Ageing Res. Rev., 8 (2009), pp. 18–30
- [188] P.L. Molyneaux, T.M. Maher; The role of infection in the pathogenesis of idiopathic pulmonary fibrosis; Eur. Respir. Rev., 22 (2013), pp. 376–381
- [189] B.A. Lidbury, I.A. Ramshaw, M.S. Rolph, W.B. Cowden; The antiviral activity of tumour necrosis factor on herpes simplex virus type 1: role for a butylated hydroxyanisole sensitive factor; Arch. Virol., 140 (1995), pp. 703–719
- [190] T. Valyi-Nagy, S.J. Olson, K. Valyi-Nagy, T.J. Montine, T.S. Dermody; Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons; Virology, 278 (2000), pp. 309–321
- [191] V.P. Skulachev; Possible role of reactive oxygen species in antiviral defense; Biochemistry, 63 (1998), pp. 1438–1440
- [192] F. Calabrese, A. Kipar, F. Lunardi, E. Balestro, E. Perissinotto, E. Rossi, N. Nannini, G. Marulli, J.P. Stewart, F. Rea; Herpes virus infection is associated with vascular remodeling and pulmonary hypertension in idiopathic pulmonary fibrosis; PLoS One, 8 (2013), p. e55715
- [193] H. Bruunsgaard, M. Pedersen, B.K. Pedersen; Aging and proinflammatory cytokines; Curr. Opin. Hematol., 8 (2001), pp. 131–136
- [194] A.R. Hipkiss; Accumulation of altered proteins and ageing: causes and effects; Exp. Gerontol., 41 (2006), pp. 464–473
- [195] C.N. Oliver, B.W. Ahn, E.J. Moerman, S. Goldstein, E.R. Stadtman; Age-related changes in oxidized proteins; J. Biol. Chem., 262 (1987), pp. 5488–5491
- [196] R. Aeschbach, R. Amado, H. Neukom; Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues; Biochim Biophys. Acta, 439 (1976), pp. 292–301
- [197] S. Reeg, T. Grune; Protein Oxidation in Aging: Does It Play a Role in Aging Progression?; Antioxid. Redox Signal, 23 (2015), pp. 239–255
- [198] R. Issa, X. Zhou, C.M. Constandinou, J. Fallowfield, H. Millward-Sadler, M.D. Gaca, E. Sands, I. Suliman, N. Trim, A. Knorr, M.J. Arthur, R.C. Benyon, J.P. Iredale; Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking; Gastroenterology, 126 (2004), pp. 1795–1808
- [199] J.M. Larios, R. Budhiraja, B.L. Fanburg, V.J. Thannickal; Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts; J. Biol. Chem., 276 (2001), pp. 17437–17441
- [200] S. Pennathur, A. Vivekanandan-Giri, M.L. Locy, T. Kulkarni, D. Zhi, L. Zeng, J. Byun, J.A. de Andrade, V.J. Thannickal; Oxidative modifications of protein tyrosyl residues are increased in plasma of human subjects with interstitial lung disease; Am. J. Respir. Crit. Care Med., 193 (2016), pp. 861–868
- [201] S.I. Hagiwara, Y. Ishii, S. Kitamura; Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice; Am. J. Respir. Crit. Care Med., 162 (2000), pp. 225–231
- [202] A. Serrano-Mollar, D. Closa, N. Prats, S. Blesa, M. Martinez-Losa, J. Cortijo, J.M. Estrela, E.J. Morcillo, O. Bulbena; In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats; Br. J. Pharmacol., 138 (2003), pp. 1037–1048
- [203] Y. Muramatsu, K. Sugino, F. Ishida, J. Tatebe, T. Morita, S. Homma; Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis; Respir. Invest., 54 (2016), pp. 170–178
- [204] F.J. N., Martinez, J.A. de Andrade, K.J. Anstrom, T.E. King Jr., G. Raghu; Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis; (Idiopathic Pulmonary) Fibrosis Clinical Research N Engl. J. Med, 370 (2014), pp. 2093–2101
- [205] M. Myllarniemi, R. Kaarteenaho; Pharmacological treatment of idiopathic pulmonary fibrosis - preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine; Eur. Clin. Respir. J., 2 (2015)
- [206] J.M. Oldham, S.F. Ma, F.J. Martinez, K.J. Anstrom, G. Raghu, D.A. Schwartz, E. Valenzi, L. Witt, C. Lee, R. Vij, Y. Huang, M.E. Strek, I. Noth, I.P. Investigators; TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis; Am. J. Respir. Crit. Care Med., 192 (2015), pp. 1475–1482
- [207] X. He, L. Wang, G. Szklarz, Y. Bi, Q. Ma; Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway; J. Pharmacol. Exp. Ther., 342 (2012), pp. 81–90
- [208] G. Sener, N. Topaloglu, A.O. Sehirli, F. Ercan, N. Gedik; Resveratrol alleviates bleomycin-induced lung injury in rats; Pulm. Pharmacol. Ther., 20 (2007), pp. 642–649
- [209] S.N. Zucker, et al.; Nrf2 amplifies oxidative stress via induction of Klf9; Mol. Cell., 53 (2014), pp. 916–928
Document information
Published on 20/10/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?